D(+)-Glucose
Synonym(s):D -(+)-Glucose;Dextrose;Dextrosum (Glucosum);D-Glucopyranose;D-Glucose, Corn sugar
- CAS NO.:50-99-7
- Empirical Formula: C6H12O6
- Molecular Weight: 180.16
- MDL number: MFCD00063684
- EINECS: 200-075-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
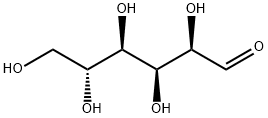
What is D(+)-Glucose?
Absorption
D(+)-Glucose can be efficiently broken down into their simpler sugar units by the action of pancreatic and intestinal glycosidases, as well as by the intestinal flora. This process is essential for the absorption of glucose, a primary source of energy for the body. The transport of glucose from the intestine into the bloodstream is predominantly facilitated by two specific transport proteins: the sodium-dependent glucose transporter SGLT1 and the glucose transporter GLUT2, also known as SLC2A2. SGLT1 is strategically located in the apical membrane of the intestinal wall, where it actively transports glucose against a concentration gradient, utilizing the sodium gradient.
Toxicity
Oral LD50 value of D(+)-Glucose in rats is 25800mg/kg. The administration of glucose infusions can cause fluid and solute overloading resulting in dilution of the serum electrolyte concentrations, overhydration, congested states, or pulmonary edema. Hypersensitivity reactions may also occur including anaphylactic/anaphylactoid reactions from oral tablets and intravenous infusions.
Description
D(+)-Glucose, the most prevalent simple sugar found in nature, serves as a fundamental building block for a variety of carbohydrates, including the disaccharides sucrose and lactose, as well as more complex oligo- and polysaccharides. Remarkably, it is the sole sugar component in both cellulose and starch, which are essential structural and storage carbohydrates in plants, respectively. The production of D-glucose occurs through different processes in animals and plants: glycogenolysis in animals, where glycogen is broken down to release glucose, and photosynthesis in plants, where sunlight, carbon dioxide, and water are converted into glucose and oxygen. This sugar is utilized by both kingdoms as a primary energy source, highlighting its crucial role in sustaining life. In chemical structures, D-glucose is often represented in its open chain form; however, in an aqueous solution, over 99% of D-glucose molecules exist in one of two closed pyranose ring forms, with α-D-glucopyranose being one of the commonly depicted structures.
Chemical properties
White or almost white, crystalline powder.
Originator
Dextrose,Wockhardt Ltd.,India
History
D(+)-Glucose is the most important and predominant monosaccharide found in nature. It was isolated from raisins by Andreas Sigismund Marggraf (1709–1782) in 1747, and in 1838, Jean-Baptiste-André Dumas (1800–1884) adopted the name glucose from the Greek word glycos meaning sweet. Emil Fischer (1852–1919) determined the structure of glucose in the late 19th century. Glucose also goes by the names dextrose (from its ability to rotate polarized light to the right), grape sugar, and blood sugar. The term blood sugar indicates that glucose is the primary sugar dissolved in blood. Glucose’s abundant hydroxyl groups enable extensive hydrogen bonding, and so glucose is highly soluble in water.
The Uses of D(+)-Glucose
D(+)-Glucose is a primary source of energy for living organismsis a reducing sugar and produces a high-temperature browning effect in baked goods. It is used in ice cream, bakery products, and confections. It is also termed corn sugar.
Indications
Glucose pharmaceutical formulations (oral tablets, injections) are indicated for caloric supply and carbohydrate supplementation in case of nutrient deprivation. It is also used for metabolic disorders such as hypoglycemia.
Background
D(+)-Glucose is a simple sugar (monosaccharide) generated during phosynthesis involving water, carbon and sunlight in plants. It is produced in humans via hepatic gluconeogenesis and breakdown of polymeric glucose forms (glycogenolysis). It circulates in human circulation as blood glucose and acts as an essential energy source for many organisms through aerobic or anaerobic respiration and fermentation. It is primarily stored as starch in plants and glycogen in animals to be used in various metabolic processes in the cellular level. Its aldohexose stereoisomer, dextrose or D-glucose, is the most commonly occurring isomer of glucose in nature.
Definition
ChEBI: The open chain form of D-glucose.
Production
D(+)-Glucose is produced industrially from starch by enzymatic hydrolysis using glucose amylase or by the use of acids. Enzymatic hydrolysis has largely displaced acid-catalyzed hydrolysis reactions.
brand name
Cartose (Sterling Winthrop) Dextrose.
Therapeutic Function
Sugar supplement
General Description
Watery odorless colorless liquid. Denser than water and soluble in water. Hence sinks in and mixes with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
A weak reducing agent.
Health Hazard
No toxicity
Pharmacokinetics
Blood glucose is an obligatory energy source for humans involved in various cellular activities, and it also acts as a signaling molecule for diverse glucose-sensing molecules and proteins. Glucose undergoes oxidation into carbon dioxide, water, and yields energy molecules in the process of glycolysis and subsequent citric cycle and oxidative phosphorylation. Glucose is readily converted into fat in the body which can be used as a source of energy as required. Under a similar conversion into storage of energy, glucose is stored in the liver and muscles as glycogen. Glucose stores are mobilized in a regulated manner, depending on the tissues' metabolic demands. Oral glucose tablets or injections serve to increase the supply of glucose and oral glucose administration is more effective in stimulating insulin secretion because it stimulates the incretin hormones from the gut, which promotes insulin secretion.
Safety Profile
Mildly toxic by ingestion the D(+)-Glucose. An experimental teratogen. Experi mental reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Potentially explosive reaction with potassium nitrate + sodium peroxide when heated in a sealed container. Uxtures with alkali release carbon monoxide when heated. When heated to decomposition it emits acrid smoke and irritating fumes.
Metabolism
D(+)-Glucose can undergo aerobic oxidation in conjunction with the synthesis of energy molecules. Glycolysis is the initial stage of glucose metabolism where one glucose molecule is degraded into two molecules of pyruvate via substrate-level phosphorylation. These products are transported to the mitochondria where they are further oxidized into oxygen and carbon dioxide.
Purification Methods
Crystallise -D-glucose from hot glacial acetic acid or pyridine. Traces of solvent are removed by drying in a vacuum oven at 75o for >3hours. [Gottfried Adv Carbohydr Chem 5 127 1950, Kjaer & Lindberg Acta Chem Scand 1 3 1713 1959, Whistler & Miller Methods in Carbohydrate Chemistry I 1301962, Academic Press, Beilstein 1 IV 4306.] [For equilibrium forms see Angyal Adv Carbohydr Chem 42 15 1984, Angyal & Pickles Aust J Chem 25 1711 1972.]
Properties of D(+)-Glucose
| Melting point: | 150-152 °C(lit.) |
| Boiling point: | 232.96°C (rough estimate) |
| alpha | 52.75 º (c=10, H2O, NH4OH 25 ºC) |
| Density | 1.5440 |
| refractive index | 53 ° (C=10, H2O) |
| storage temp. | room temp |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Crystalline Powder |
| pka | pKa 12.43(H2O,t = 18,)(Approximate) |
| color | White |
| Odor | Odorless |
| PH | 5.0-7.0 (25℃, 1M in H2O) |
| PH Range | 5.9 |
| optical activity | [α]25/D +52.5 to +53.0°(lit.) |
| Water Solubility | Soluble |
| λmax | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,4459 |
| BRN | 1281608 |
| Stability: | Stable. Substances to be avoided include strong oxidizing agents. Combustible. |
| CAS DataBase Reference | 50-99-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Glucose(50-99-7) |
| EPA Substance Registry System | Dextrose (50-99-7) |
Safety information for D(+)-Glucose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H227:Flammable liquids H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for D(+)-Glucose
| InChIKey | WQZGKKKJIJFFOK-DVKNGEFBSA-N |
D(+)-Glucose manufacturer
Vinayak Enterprise
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Dextrose 98%View Details
Dextrose 98%View Details -
 110187-43-3 98%View Details
110187-43-3 98%View Details
110187-43-3 -
 Dextroseanhydrous 99%View Details
Dextroseanhydrous 99%View Details -
 Dextrose for cell culture CAS 50-99-7View Details
Dextrose for cell culture CAS 50-99-7View Details
50-99-7 -
 Dextrose ACS CAS 50-99-7View Details
Dextrose ACS CAS 50-99-7View Details
50-99-7 -
 Dextrose CAS 50-99-7View Details
Dextrose CAS 50-99-7View Details
50-99-7 -
 DEXTROSE (Anhydrous) Extra Pure LR (5kg)View Details
DEXTROSE (Anhydrous) Extra Pure LR (5kg)View Details
50-99-7 -
 Unieversal Dextrose Anhydrous Powder, For Industrial, Grade: Food GradeView Details
Unieversal Dextrose Anhydrous Powder, For Industrial, Grade: Food GradeView Details
50-99-7
