Acesulfame potassium
Synonym(s):6-Methyl-1,2,3-oxathiazin-4(3H)-one 2,2-dioxide potassium salt;Acesulfame K
- CAS NO.:55589-62-3
- Empirical Formula: C4H4KNO4S
- Molecular Weight: 201.24
- MDL number: MFCD00043833
- EINECS: 259-715-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:38
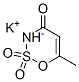
What is Acesulfame potassium?
Description
Acesulfame potassium is 200 times sweeter than sucrose, but it is not metabolized in the human body. Acetoacetamide, a breakdown product, has been shown to affect the thyroid in animal studies; however, there is some doubt as to whether this product is likely to form during normal metabolism.
Description
Acesulfame potassium is approved for use in food as a sweetener. It is included in the ingredient list on the food label as acesulfame K, acesulfame potassium, or Ace-K. Acesulfame potassium (Ace-K) is regulates as a food additive. The FDA approved acesulfame potassium for use in specific food and beverage categories in 1988 and in 2003 approved it as a general-purpose sweetener and flavor enhancer in food, except in meat and poultry, under certain conditions of use. It is widely used in the food industry, such as mouthwash and soft drinks. However, some researches have found that it does harm to the liver and nervous system[1-2].
Chemical properties
White to Off-White Solid
Chemical properties
Acesulfame potassium occurs as a colorless to white-colored, odorless, crystalline powder with an intensely sweet taste.
Originator
Acesulfame Potassium ,Hoechst
History
Acesulfame-K, the potassium salt of acesulfame, is a sweetener that resembles saccharin in structure and taste profile. 5,6-Dimethyl-1,2,3-oxathiazine-4(3H)-one 2,2-dioxide, the first of many sweet compounds belonging to the dihydrooxathiazinone dioxide class, was discovered accidentally in 1967. From these many sweet compounds, acesulfame was chosen for commercialization. To improve water solubility, the potassium salt was made. Acesulfame-K (Sunett) was approved for dry product use in the United States in 1988 and in Canada in October, 1994. In 2003, acesulfame-K was approved as a general purposes sweetener by the FDA.
The Uses of Acesulfame potassium
'New generation', heat-stable sweetener that has not been suspected to cause cancer nor be genotoxic. Allelic variation of the Tas1r3 gene affects behavioral taste responses to this molecule, suggesting that it is a T1R3 receptor ligand.
The Uses of Acesulfame potassium
Potassium salt as sweetener for foods, cosmetics.
The Uses of Acesulfame potassium
Acesulfame-K is the potassium salt of 6-methyl-l,2,3-oxathiazin-4(3H)-
one-2,2-dioxide. This sweetener was discovered in Germany and was
first approved by the FDA in 1988 for use as a nonnutritive sweetener.
The complex chemical name of this substance led to the creation of the
trademark common name, acesulfame-K, which is based on its following
relationships to acetocetic acid and sulfanic acid, and to its potassium
salt nature.
Acesulfame-K is 200 times as sweet as sugar and is not metabolized
and is thus noncaloric. It is exceptionally stable at elevated temperatures
encountered in baking, and it is also stable in acidic products, such as
carbonated soft drinks. It has a synergistic effect when mixed with other
low-calorie sweetners, such as aspartame. Common applications of
acesulfame-K are table uses, chewing gums, beverages, foods, bakery
products, confectionary, oral hygiene products, and pharmaceuticals.
What are the applications of Application
Acesulfame Potassium is a synthetic sweetner
Production Methods
Acesulfame potassium is synthesized from acetoacetic acid tertbutyl
ester and fluorosulfonyl isocyanate. The resulting compound
is transformed to fluorosulfonyl acetoacetic acid amide, which is
then cyclized in the presence of potassium hydroxide to form the
oxathiazinone dioxide ring system. Because of the strong acidity of
this compound, the potassium salt is produced directly.
An alternative synthesis route for acesulfame potassium starts
with the reaction between diketene and amidosulfonic acid. In the
presence of dehydrating agents, and after neutralization with
potassium hydroxide, acesulfame potassium is formed.
Production Methods
The principal commercial process for acesulfame-K is depicted below:

Definition
ChEBI: Acesulfame k is a sulfuric acid derivative.
Manufacturing Process
80 g (1.096 mol) of dimethylethylamine were added drop-wise, with cooling,
to 80 g (0.825 mol) of sulfamic acid suspended in 500 ml of glacial acetic
acid. When dissolution was complete, 80 ml (1.038 mol) of diketene were
added, while cooling at 25°-35°C. After 16 hours, the mixture was evaporated
and the residue was stirred with acetone, whereupon crystallization of
dimethylethylammonium acetoacetamide-N-sulfonate took place. Yield: 110 g
(43%), melting point 73°-75°C.
12.7 g (50 mmol) of dimethylethylammonium acetoacetamide-N-sulfonate in
110 ml of methylene chloride were added drop-wise to 8 ml (200 mmol) of
liquid SO3 in 100 ml of CH2Cl2 at -30°C, stirring vigorously, within 60
minutes. 30 minutes later, 50 ml of ethyl acetate and 50 g of ice were added
to the solution. The organic phase was separated off, and the aqueous phase
was extracted twice more with ethyl acetate. The combined organic phases
were dried over sodium sulfate, evaporated and the residue was dissolved in
methanol. On neutralization of the solution with methanolic KOH, the
potassium salt of 6-methyl-3,4-dihydro-1,2,3-oxathiazin-4-one 2,2-dioxide
precipitated out. Yield: 7.3 g (73%). The product was detected by thinlayer
chromatography; the structure of it was confirmed with IR spectrum.
brand name
Sunett? and Sweet One?
Therapeutic Function
Pharmaceutic aid
Pharmaceutical Applications
Acesulfame potassium is used as an intense sweetening agent in
cosmetics, foods, beverage products, table-top sweeteners, vitamin
and pharmaceutical preparations, including powder mixes, tablets,
and liquid products. It is widely used as a sugar substitute in
compounded formulations,and as a toothpaste sweetener.
The approximate sweetening power is 180–200 times that of
sucrose, similar to aspartame, about one-third as sweet as sucralose,
one-half as sweet as sodium saccharin, and about 4-5 times sweeter
than sodium cyclamate.It enhances flavor systems and can be
used to mask some unpleasant taste characteristics.
Biological Activity
In rats, Acesulfame potassium (AceK) consumption from in-utero to post-weaning stages accelerated puberty onset, accompanied by increased brain gonadotropin-releasing hormone (GnRH) expression. Intracerebroventricular AceK injection also induced early puberty onset in rats. In N44 hypothalamic neuron cells, AceK treatment increased reactive oxygen species production, which led to protein kinase A (PKA) activation and increased GnRH expression[1].
Safety Profile
When heated to decompositionemits toxic fumes of SOx.
Safety
Acesulfame potassium is widely used in beverages, cosmetics, foods,
and pharmaceutical formulations, and is generally regarded as a
relatively nontoxic and nonirritant material. Pharmacokinetic
studies have shown that acesulfame potassium is not metabolized
and is rapidly excreted unchanged in the urine. Long-term feeding
studies in rats and dogs showed no evidence to suggest acesulfame
potassium is mutagenic or carcinogenic.
The WHO has set an acceptable daily intake for acesulfame
potassium of up to 15 mg/kg body-weight.The Scientific
Committee for Foods of the European Union has set a daily intake
value of up to 9 mg/kg of body-weight.
LD50 (rat, IP): 2.2 g/kg
LD50 (rat, oral): 6.9–8.0 g/kg
Storage
Acesulfame potassium possesses good stability. In the bulk form it
shows no sign of decomposition at ambient temperature over many
years. In aqueous solutions (pH 3.0–3.5 at 208℃) no reduction in
sweetness was observed over a period of approximately 2 years.
Stability at elevated temperatures is good, although some decomposition
was noted following storage at 408℃ for several months.
Sterilization and pasteurization do not affect the taste of acesulfame
potassium.
The bulk material should be stored in a well-closed container in a
cool, dry place and protected from light.
Regulatory Status
Included in the FDA Inactive Ingredients Database for oral and sublingual preparations. Included in the Canadian List of Acceptable Non-medicinal Ingredients. Accepted for use in Europe as a food additive. It is also accepted for use in certain food products in the USA and several countries in Central and South America, the Middle East, Africa, Asia, and Australia.
References
[1] Wu H, et al. Consumption of the nonnutritive sweetener acesulfame potassium increases central precocious puberty risk. Journal of Hazardous Materials, 2023; 461: 132529.
[2] Duan X, et al. Detection of acesulfame potassium in mouthwash based on surface-enhanced Raman spectroscopy. Optical Engineering, 2018; 57: 057102 .
Properties of Acesulfame potassium
| Melting point: | 229-232°C (dec.) |
| Boiling point: | 210℃[at 101 325 Pa] |
| Density | (solid) 1.81 g/cm3; d (bulk) 1.1-1.3 kg/dm3 |
| vapor pressure | 0.291Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Soluble in water, very slightly soluble in acetone and in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| color | White crystalline solid |
| Odor | odorless with sweet taste |
| Water Solubility | almost transparency |
| Merck | 14,37 |
| BRN | 3637857 |
| CAS DataBase Reference | 55589-62-3(CAS DataBase Reference) |
| EPA Substance Registry System | Acesulfame-potassium (55589-62-3) |
Safety information for Acesulfame potassium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Acesulfame potassium
Acesulfame potassium manufacturer
Gangwal Healthcare Pvt Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Acesulfame K CAS 55589-62-3View Details
Acesulfame K CAS 55589-62-3View Details
55589-62-3 -
 Acesulfame potassium CAS 55589-62-3View Details
Acesulfame potassium CAS 55589-62-3View Details
55589-62-3 -
 Acesulfame K 95.00% CAS 55589-62-3View Details
Acesulfame K 95.00% CAS 55589-62-3View Details
55589-62-3 -
 Acesulfame K CAS 55589-62-3View Details
Acesulfame K CAS 55589-62-3View Details
55589-62-3 -
 Acesulfame K >98% (HPLC) CAS 55589-62-3View Details
Acesulfame K >98% (HPLC) CAS 55589-62-3View Details
55589-62-3 -
 Acesulfame Potassium, Packaging Size: 25 kg, For FoodView Details
Acesulfame Potassium, Packaging Size: 25 kg, For FoodView Details
55589-62-3 -
 Acesulfame Potassium, Packaging Size: 25 kgView Details
Acesulfame Potassium, Packaging Size: 25 kgView Details
55589-62-3 -
 Acesulfame Potassium (AK Sugar) CAS 55589-62-3View Details
Acesulfame Potassium (AK Sugar) CAS 55589-62-3View Details
55589-62-3
