Modafinil
Synonym(s):2-[(Diphenylmethyl)sulfinyl]-acetamide
- CAS NO.:68693-11-8
- Empirical Formula: C15H15NO2S
- Molecular Weight: 273.35
- MDL number: MFCD00868082
- EINECS: 640-968-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
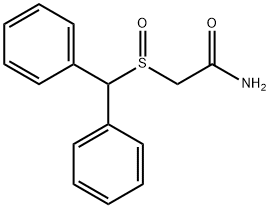
What is Modafinil?
Absorption
Rapid following oral administration.
Description
Modafinil, a centrally active α1-adrenergic agonist, was marketed in France as a psychostimulant for the treatment of narcolepsy and idiopathic hypersomnia including Gelineau's syndrome. In monkeys, modafinil was found to induce potent behavioral stimulation and awakening without stereotyped behavior. In narcoleptic patients, modafinil reduces the daily number of sleep attacks significantly and markedly improves performance. The mechanism of action for its locomotor effects was reported to be due to the stimulation of the central α1-adrenergic system, opposite to amphetamine and methylphenidate, which act mainly by dopaminergic mechanisms. Modafinil has been reported to exhibit minimal peripheral side effects at therapeutic doses and appears to have low abuse potential. The use of modafinil in the treatment of cerebral infarction and ischemia has been claimed.
Description
Modafinil is a central nervous system stimulant that is US Food and Drug Administration-approved for treating narcolepsy. Its ability to induce "wakefulness" in people subject to fatigue is being studied, but modafinil has not yet been approved for this use.
Chemical properties
Crystalline Solid
Originator
Lafon (France)
The Uses of Modafinil
Modafinil is an α-1-adrenergic agonist. Modafinil is a CNS stimulant; psychostimulant that displays neuroprotective and antiparkinsonian activity in a primate model of Parkinson's disease.
The Uses of Modafinil
A central nervous system vigilance promoting agent. Possesses neuroprotective properties. This is a controlled substance (stimulant)
The Uses of Modafinil
ACE inhibitor, antihypertensive
Background
Modafinil is a stimulant drug marketed as a 'wakefulness promoting agent' and is one of the stimulants used in the treatment of narcolepsy. Narcolepsy is caused by dysfunction of a family of wakefulness-promoting and sleep-suppressing peptides, the orexins, whose neurons are activated by modafinil. The prexin neuron activation is associated with psychoactivation and euphoria. The exact mechanism of action is unclear, although in vitro studies have shown it to inhibit the reuptake of dopamine by binding to the dopamine reuptake pump, and lead to an increase in extracellular dopamine. Modafinil activates glutamatergic circuits while inhibiting GABA.
Indications
To improve wakefulness in patients with excessive daytime sleepiness (EDS) associated with narcolepsy.
Definition
ChEBI: 2-[(diphenylmethyl)sulfinyl]acetamide is a sulfoxide that is dimethylsulfoxide in which two hydrogens attached to one of the methyl groups are replaced by phenyl groups, while one hydrogen attached to the other methyl group is replaced by a carbamoyl (aminocarbonyl) group. It is a sulfoxide and a monocarboxylic acid amide.
Manufacturing Process
To 19.5 g (0.076 mol) of benzhydrylthioacetic acid in 110 ml of benzene was
added drop by drop 19 ml of thionyl chloride. The mixture was refluxed 1
hour, benzene and the excess thionyl chloride was evaporated. A clear orange
oil of benzhydrylthioacetyl chloride is obtained.
The benzhydrylthioacetyl chloride in 100 ml of methylene chloride was added
drop by drop to 35 ml of ammonia in 40 ml of water. Once the addition is
complete, the organic phase is washed with a dilute solution of soda and dried
over Na2SO4, the solvent is evaporated and the residue is taken up in
diisopropyl ether, the benzhydrylthioacetamide is crystallised. 16.8 g of
product (yield 86%) are obtained. Melting point 110°C.
14.39 g (0.056) of benzhydrylthioacetamide are placed in a balloon flask and
60 ml of acetic acid and 5.6 ml of H2O2 are added. The mixture is left for one
night at 40°C and about 200 ml of water are then added; the CRL 40476
crystallises. By recrystallisation from methanol 11.2 g of
benzhydrylsulphinylacetamide are obtained. Yield: 73%, melting point 164-
166°C.
The synthesis of benzhydrylsulphinylacetamide (CRL 40476) on an industrial
scale.
1.003 kg of thiourea is dissolved in 5.72 L of 48% hydrobromic acid and
0.880 L of water in a 20 L reaction vessel. The mixture is heated to 60°C and
2.024 kg of benzhydrol are introduced. The temperature is increased to 95°C
and the contents of the vessel are allowed to cool to room temperature. The
crystals are filtered off and washed with water. They are made into a paste
again in 5.5 L of water and this is introduced into a 20 L reaction vessel with
3.5 L of soda lye (d = 1.33). The mixture is heated to 70°C and 1144 g of
chloroacetic acid dissolved in 2.2 L of water are passed in slowly. After cooling
the benzhydrylthioacetic acid is obtained, but is not isolated.
1.430 L of hydrogen peroxide are passed in over 3 hours at 30°C into the
above reaction mixture. 22 L of water are then passed in, the insoluble
material is filtered off and acidification is carried out with hydrochloric acid (d
= 1.18). Filtration, washing with water to reform a paste and drying without
heat are carried out. In this way, the benzhydrylsulphinylacetic acid is
obtained.
The above acid is placed in a 20 L reaction vessel with 6 L of water. 1.1 liters
of soda lye (d = 1.33) and 1.848 kg of sodium bicarbonate are added. 2.1 L
of dimethyl sulfate are added. After one hour, crystallisation is induced.
Filtration, drying without heat and washing are carried out. Methyl
benzhydrylsulphinylacetate is obtained.
1 kg of methyl benzhydrylsulphinylacetate is dissolved in 3.5 liters of
anhydrous methanol in a 10-liter balloon flask. NH3 is bubbled in at a high
rate of flow for 1 hour, and then left in contact for 4 hours. Filtration, drying
without heat and washing with water are then carried out. By recrystallisation
from a mixture of water and methanol (4:1) and then from a mixture of water
and methanol (9:1) and drying under reduced pressure, CRL 40476 is
obtained in the form of a white crystalline powder; melting point 164-166°C.
Total yield (calculated from the benzhydrol): 41%.
brand name
Provigil (Cephalon);Modiodal.
Therapeutic Function
Psychostimulant
General Description
Modafinil (Provigil) has overall wakefulness-promotingproperties similar to those of central sympathomimetics. It isconsidered an atypical α1-norepinephrine (NE) receptorstimulant and is used to treat daytime sleepiness in narcolepsypatients. Adverse reactions at therapeutic doses arereportedly not severe and may include nervousness, anxiety,and insomnia. Modafinil is used by oral administration.
Biological Activity
Psychostimulant that displays neuroprotective and antiparkinsonian activity in a primate model of Parkinson's disease. Promotes vigilence and wakefulness without the central and peripheral side effects associated with conventional dopaminergic psychostimulants. Mechanism of action is not fully understood, possibly via modulation of catecholamine/GABAergic neurotransmission.
Pharmacokinetics
Modafinil is a stimulant drug marketed as a 'wakefulness promoting agent' and is one of the stimulants used in the treatment of narcolepsy. Narcolepsy is caused by dysfunction of a family of wakefulness-promoting and sleep-suppressing peptides, the orexins, whose neurons are activated by modafinil. The prexin neuron activation is associated with psychoactivation and euphoria. Modafinil is not indicated for complaints of lack of energy or fatigue; but it appears to be very helpful for some patients. Also, it has been used in the treatment of hypersomnia, a disorder in which patients lack the capacity for meaningful sleep and may require ten or more hours per day. Recent studies have have found that modafinil may help recovering cocaine addicts fight their addiction.
Clinical Use
Excessive daytime drowsiness associated with narcolepsy and obstructive sleep apnoea
Drug interactions
Potentially hazardous interactions with other drugs
Ciclosporin: reduced ciclosporin concentration.
Cytotoxics: concentration of bosutinib possibly
reduced - avoid.
Guanfacine: concentration of guanfacine possibly
reduced - avoid.
Oestrogens: metabolism accelerated (reduced
contraceptive effect).
Ulipristal: possibly reduces contraceptive effect of
ulipristal - avoid
Metabolism
Hepatic
Metabolism
Metabolised in the liver, partly by the cytochrome P450 isoenzymes CYP3A4 and CYP3A5; two major metabolites have been identified: acid modafinil and modafinil sulfone, both of which are inactive. Excretion is mainly through the kidneys.
Storage
+4°C
Properties of Modafinil
| Melting point: | 164-166°C |
| Boiling point: | 559.1±50.0 °C(Predicted) |
| Density | 1.283±0.06 g/cm3(Predicted) |
| Flash point: | 2℃ |
| storage temp. | Store at +4°C |
| solubility | DMSO: 18 mg/mL, soluble |
| form | white solid |
| pka | 14.88±0.40(Predicted) |
| color | white |
| CAS DataBase Reference | 68693-11-8(CAS DataBase Reference) |
Safety information for Modafinil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Modafinil
Modafinil manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Modafinil >95% CAS 68693-11-8View Details
Modafinil >95% CAS 68693-11-8View Details
68693-11-8 -
 Modafinil Powder APIView Details
Modafinil Powder APIView Details
68693-11-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
