N-Acetylsulfanilyl chloride
Synonym(s):4-Acetamidobenzenesulfonyl chloride
- CAS NO.:121-60-8
- Empirical Formula: C8H8ClNO3S
- Molecular Weight: 233.67
- MDL number: MFCD00007442
- EINECS: 204-485-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
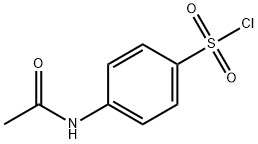
What is N-Acetylsulfanilyl chloride?
Chemical properties
OFF-WHITE TO SLIGHTLY GREY GRANULAR CRYST. POWDER
The Uses of N-Acetylsulfanilyl chloride
N-Acetylsulfanilyl chloride is used?in the preparation of sulfanilamide and its derivatives which are intermediates to produce sulfa drugs. They are used in the prevention and treatment of bacterial infections, diabetes mellitus, edema, hypertension, and gout. It is also used as a Pharma raw material. N-Acetylsulfanilyl chloride widely used in the fields of dye, medicine, mainly used for preparation of Sulfanilamide, Sulfanilylureal, Sulfatolamide, Sulphathiourea , Sulfaguanidine and Sulfacetamide and etc.
The Uses of N-Acetylsulfanilyl chloride
A sulfanilamide derivative of Chitosan
The Uses of N-Acetylsulfanilyl chloride
Intermediate in the preparation of sulfanilamide and its derivatives.
Production Methods
Acetanilide is introduced into stirred chlorosulfuric acid solution at 20 ℃. The solution is then heated to 55 ℃ and kept at this temperature for 1.5 h. The solution is cooled to 20 ℃ and introduced as a thin jet into water, the temperature of which is kept at 0 – 5 ℃ by external cooling. The precipitated sulfochloride is filtered and washed with cold water until neutral to Congo Red. Yield is 80 – 82%. The crude product contains about 60% water; the dried product melts at 144 – 147 ℃. About 1 – 2% of bis(acetylaminophenyl) sulfone is obtained as a byproduct. If thionyl chloride is added, less chlorosulfuric acid is needed because it can be replaced partly byoleum. The product is stable for several days if kept in a cool place. It is generally used directly in the moist state. 4-(Acetylamino)benzenesulfonyl chloride is an important intermediate in the manufacture of sulfonamides and in the synthesis of parabase ester.
Biotechnological Production
After more than three decades of strain and process optimization, the 2KGA fermentation by K. vulgare has reached a performance level that makes it increasingly difficult to achieve further cost-relevant improvements. Instead, opportunities can be seen in the succeeding step of 2KGA rearrangement to ascorbic acid, which still follows the same concept as laid out in the 1930s by Reichstein and Grüssner. This chemical step contributes significantly to the overall process costs. A process Industrial Production of L-Ascorbic Acid (Vitamin C) and D-Isoascorbic Acid 171 concept that could convert sorbitol directly to ascorbic acid would therefore be most attractive. In theory, this could build on the established 2KGA fermentation with an enzyme-catalyzed 2KGA to Asc rearrangement (2,6-hemiacetal to 1,4- lactone) as extension. Ab initio energy calculations as well as experimental results (own unpublished results) indicate that in aqueous environment, Asc is thermodynamically far more stable than 2KGA and (nearly) quantitative conversion should be possible. However, no enzyme efficiently catalyzing this reaction has so far been identified. The few publications of enzyme catalysis for this reaction so far shows only trace activity and no significant improvements have been reported. 2KGA may represent a kinetic trap in an aqueous environment and biotechnological reaction pathways all the way to Asc may need to avoid 2KGA. Accordingly, 2KGA is also not part of natural biosynthetic routes, where Asc formation directly results from the oxidation of precursor molecules with appropriately preformed 1,4-lactone linkage (L-gulono-1,4-lactone in animals, L-galactono-1,4-lactone in plants). Enzymes converting L-gulono-1,4-lactone to Asc are also known from bacteria, even from Ketogulonicigenium. The biochemical description of the Ketogulonicigenium enzyme indicates that it belongs to the family of heterotrimeric periplasmic flavohemoproteins, of which several can be found in the published Ketogulonicigenium genomes. Besides sharing the same FAD cofactor, these enzymes bear no similarity to the mammalian gulono-1,4- lactone dehydrogenase. The use of these natural or nature-like Asc-forming enzymatic steps in biotechnological production processes is so far precluded by the rare nature of these L-sugar-derived lactone precursor molecules and the lack of efficient production methods for these compounds. It was, therefore, a tantalizing discovery when Asc formation directly from L-sorbosone, the intermediate of the efficient 2KGA formation route, was identified in those two species already in the focus for 2KGA production for decades: K. vulgare and G. oxydans. Besides an earlier report of L-sorbosone to Asc activity derived from plant tissue , which did not see consolidating follow-ups, the above observations are the first evidence of biological Asc formation from a molecule other than a 1,4-lactone.
General Description
L-hydroxyproline has been derivatized with N-acetylsulfanilyl chloride and 5-chlorovaleric acid during the synthesis of the haptens HP1 and HP2.
Flammability and Explosibility
Not classified
Safety Profile
A poison by intraperitoneal route.Moderately toxic by ingestion. When heated todecomposition it emits toxic vapors of NOx, SOx, and Cl.
Purification Methods
Crystallise the chloride from toluene, CHCl3, or ethylene dichloride. [Beilstein 14 IV 2703.]
Properties of N-Acetylsulfanilyl chloride
| Melting point: | 142-145 °C (dec.)(lit.) |
| Boiling point: | 426.8±28.0 °C(Predicted) |
| Density | 1.2977 (rough estimate) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | 1.6300 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 13.75±0.70(Predicted) |
| form | Granular Crystalline Powder or Crystals |
| color | White to cream-beige |
| Water Solubility | SLIGHTLY SOLUBLE |
| Sensitive | Moisture Sensitive |
| Merck | 14,103 |
| BRN | 746676 |
| CAS DataBase Reference | 121-60-8(CAS DataBase Reference) |
| NIST Chemistry Reference | P-acetamidobenzene sulfonyl chloride(121-60-8) |
| EPA Substance Registry System | Benzenesulfonyl chloride, 4-(acetylamino)- (121-60-8) |
Safety information for N-Acetylsulfanilyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N-Acetylsulfanilyl chloride
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)


You may like
-
 121-60-8 N-Acetylsulfanilyl chloride 98%View Details
121-60-8 N-Acetylsulfanilyl chloride 98%View Details
121-60-8 -
 4-Acetamidobenzenesulfonyl Chloride CAS 121-60-8View Details
4-Acetamidobenzenesulfonyl Chloride CAS 121-60-8View Details
121-60-8 -
 N-Acetylsulfanilyl chloride, 98% CAS 121-60-8View Details
N-Acetylsulfanilyl chloride, 98% CAS 121-60-8View Details
121-60-8 -
 N-Acetylsulfanilyl chloride 95% CAS 121-60-8View Details
N-Acetylsulfanilyl chloride 95% CAS 121-60-8View Details
121-60-8 -
 N-Acetylsulfanilyl chloride CAS 121-60-8View Details
N-Acetylsulfanilyl chloride CAS 121-60-8View Details
121-60-8 -
 N-ACETYLSULPHANILYL CHLORIDE CASView Details
N-ACETYLSULPHANILYL CHLORIDE CASView Details -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
