MIDODRINE
- CAS NO.:42794-76-3
- Empirical Formula: C12H18N2O4
- Molecular Weight: 254.28
- MDL number: MFCD00865849
- EINECS: 255-945-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
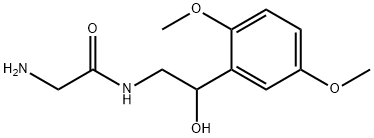
What is MIDODRINE?
Absorption
Rapidly absorbed following oral administration. The peak plasma concentrations of the prodrug, desglymidodrine, is reached about half an hour following drug administration. The metabolites reach their peak plasma concentrations at about 1 to 2 hours following drug administration. The absolute bioavailability of midodrine (measured as desglymidodrine) is 93% and is not affected by food. As desglymidodrine displays poor diffusibility across the blood-brain barrier, it is expected to have minimal effects on the central nervous system.
Toxicity
Symptoms of overdose could include hypertension, piloerection (goosebumps), a sensation of coldness and urinary retention. The single doses that would be associated with symptoms of overdosage or would be potentially life- threatening are unknown. The oral LD50 is approximately 30 to 50 mg/kg in rats, 675 mg/kg in mice, and 125 to 160 mg/kg in dogs. Desglymidodrine is dialyzable.
Originator
Gutron,Hormonchemie,W. Germany,1977
The Uses of MIDODRINE
Antihypotensive.
Background
An ethanolamine derivative that is an adrenergic alpha agonist. It is used as a vasoconstrictor agent in the treatment of hypotension.
Indications
For the treatment of symptomatic orthostatic hypotension (OH).
Definition
ChEBI: An aromatic ether that is 1,4-dimethoxybenzene which is substituted at position 2 by a 2-(glycylamino)-1-hydroxyethyl group. A direct-acting sympathomimetic with selective alpha-adrenergic agonist activity, it is used (generally as its hydro hloride salt) as a peripheral vasoconstrictor in the treatment of certain hypotensive states. The main active moiety is its major metabolite, deglymidodrine.
Manufacturing Process
19.5 parts of carbobenzoxyglycine, 7.1 parts of triethylamine and 162 parts ofdry toluene are mixed with 11.2 parts of isovaleric acid chloride at 0°C toform the mixed anhydride and the mixture is agitated for two hours at 0°C.32.4 parts of 1-(2',5'-dimethoxyphenyl)-2-aminoethanol-(1) are then added,the mixture is agitated for four hours at a temperature between 0°C and+10°C and then left to stand overnight at that temperature. A thick crystalpaste forms. The reaction product is dissolved in 450 parts of ethyl acetateand 200 parts of water. The ethyl acetate solution is separated, washed withhydrochloric acid, sodium bicarbonate solution and water, dried over sodiumsulfate and inspissated. The inspissation residue is digested with 342 parts ofxylene, the required product crystallizing out. 34.9 parts of 1-(2',5'-dimethoxyphenyl)-2-(N-carbobenzoxyglycineamido)-ethanol-(1) are obtained.66.2 parts of 1-(2',5'-dimethoxyphenyl)-2-(N-carbobenzoxyglycineamido)-ethanol-(1) are hydrogenated in the presence of 6.6 parts of palladium carbon(10%) in 2,000 parts of glacial acetic acid. When no more hydrogen isabsorbed (3 mols of hydrogen are used), hydrogenation stops. The catalyst isremoved by suction and the equivalent quantity of hydrochloric acid in ethanolis added to the filtrate with agitation. During further agitation at roomtemperature 28.6 parts of crude 1-(2',5'-dimethoxyphenyl)-2-glycineamidoethanol-(1)hydrochloride crystallize, and are isolated andrecrystallized from water-methanol for purification. 22.1 parts of pure productare obtained with a melting point of 192°C to 193°C.
An alternative synthesis route is described by Kleeman and Engel.
brand name
Orvaten (UpsherSmith); Proamatine (Shire).
Therapeutic Function
Peripheral vasotonic, Antihypotensive
Pharmacokinetics
Midodrine is a prodrug, i.e., the therapeutic effect of orally administered midodrine is due to the major metabolite desglymidodrine formed by deglycination of midodrine. Administration of midodrine results in a rise in standing, sitting, and supine systolic and diastolic blood pressure in patients with orthostatic hypotension of various etiologies. Standing systolic blood pressure is elevated by approximately 15 to 30 mmHg at 1 hour after a 10-mg dose of midodrine, with some effect persisting for 2 to 3 hours. Midodrine has no clinically significant effect on standing or supine pulse rates in patients with autonomic failure.
Metabolism
Thorough metabolic studies have not been conducted, but it appears that deglycination of midodrine to desglymidodrine takes place in many tissues, and both compounds are metabolized in part by the liver.
Properties of MIDODRINE
| Boiling point: | 529.9±50.0 °C(Predicted) |
| Density | 1.204±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Solid |
| pka | 13.47±0.20(Predicted) |
| color | White to off-white |
Safety information for MIDODRINE
Computed Descriptors for MIDODRINE
MIDODRINE manufacturer
BDR Pharmaceuticals International Pvt Ltd
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 42794-76-3 Midodrine 98%View Details
42794-76-3 Midodrine 98%View Details
42794-76-3 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1