Donepezil
- CAS NO.:120014-06-4
- Empirical Formula: C24H29NO3
- Molecular Weight: 379.49
- MDL number: MFCD00912833
- EINECS: 601-651-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
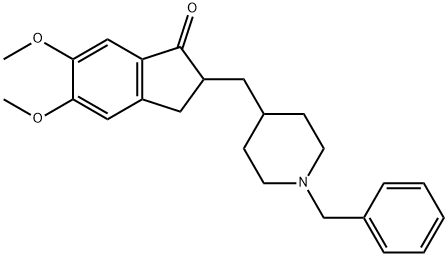
What is Donepezil?
Absorption
Donepezil is slowly absorbed via the gastrointestinal tract after oral administration. Tmax is 3 to 4 hours with a bioavailability of 100% and steady-state concentrations are attained within 15 to 21 days of administration. The Tmax in one pharmacokinetic study determined a Tmax of 4.1 ± 1.5 hours. The Cmax of 5 mg donepezil tablets is estimated to be 8.34 ng/mL, according to the Canadian monograph. The AUC of 5 mg donepezil tablets has been determined to be 221.90-225.36 ng.hr/mL.
Toxicity
LD50
The rat oral LD50 of donepezil is 32.6 mg/kg.
Overdose information
Signs and symptoms of overdose with cholinesterase inhibitors such as donepezil can include severe nausea and vomiting, bradycardia, hypotension, perspiration, seizures, muscle weakness respiratory depression, and collapse. Significant muscle weakness may result in death if the respiratory muscles are affected by donepezil overdose. To manage an overdose, anticholinergics can be employed as antidotes. Atropine at intravenous doses of 1.0 - 2.0 mg can be administered and titrated according to the clinical response. Consult the local poison control center for the most updated guidelines on the management of a donepezil overdose. Whether donepezil can be removed from the body with dialysis is unknown at this time.
Description
Donepezil is another “nonclassic,” centrally acting, reversible, noncompetitive AChEI that was approved in 1997 for treatment of mild-to-moderate AD and dementia. Its selectivity for AChE is 570- to 1,250-fold that for butyrylcholinesterase, and it also exhibits greater affinity for brain AChE than for AChE in the periphery.
The Uses of Donepezil
Acetylcholine is a neurotransmitter involved in neural signaling throughout the body. Donepezil is a reversible acetylcholinesterase inhibitor that readily crosses the blood-brain barrier to reduce the breakdown of acetylcholine. It has a half-life in circulation of about 70 hours. As acetylcholine modulates plasticity, excitability, and arousal in the central nervous system, donepezil is commonly used in the treatment of Alzheimer’s disease to improve cognition, memory, and behavior. In this way, it is intended to prevent or reverse dementia. Studies on these effects have been equivocal, indicating that more research into the therapeutic potential of donepezil is warranted.[Cayman Chemical]
The Uses of Donepezil
Donepezil is a cholinesterase inhibitors.
Indications
Donepezil, administered orally or via transdermal delivery system, is indicated for the treatment of dementia of the Alzheimer's type. It is also available as an extended-release capsule in combination with memantine for the treatment of moderate-to-severe dementia of the Alzheimer's type in patients previously stabilized on 10mg of donepezil hydrochloride once daily.
Off-label uses include the management of vascular dementia, Parkinson's Disease-associated dementia, and Lewy body dementia, amongst others.
Background
In 2016, the global burden of dementia was estimated to be 43.8 million, demonstrating a significant increase from a global prevalence of 20.2 million in 1990. Donepezil, also known as Aricept, is a piperidine derivative acetylcholinesterase inhibitor used in the management of the dementia of Alzheimer's Disease, and in some cases, is used to manage other types of dementia.
Donepezil was first approved by the FDA in 1996, and its extended-release form was approved in combination with Memantine in 2014 to manage moderate and severe forms of Alzheimer's dementia. A donepezil transdermal delivery system, Adlarity, was approved by the FDA in March 2022 for the treatment of Alzheimer's dementia. Though it does not alter the progression of Alzheimer's disease, donepezil is effective in managing the symptoms of its associated dementia.
Definition
ChEBI: Donepezil is a centrally acting reversible acetyl cholinesterase inhibitor. Its main therapeutic use is in the treatment of Alzheimer's disease where it is used to increase cortical acetylcholine.
brand name
Aricept (Eisai Medical Research).
General Description
Donepezil, (±)-2,3-dihydro-5,6-dimethoxy-2-[[1-(phenylmethyl)-4-piperidinyl]methyl]-1H-inden-1-one (Aricept), commonly referred to in the literature asE2020, is a reversible inhibitor of AChE. It is indicated forthe treatment of symptoms of mild-to-moderate Alzheimerdisease. Donepezil is approximately 96% bound to plasmaproteins, with an elimination half-life of 70 hours. It is metabolizedprincipally by the 2D6 and 3A4 isozymes of theP450 system.
Pharmacokinetics
By inhibiting the acetylcholinesterase enzyme, donepezil improves the cognitive and behavioral signs and symptoms of Alzheimer's Disease, which may include apathy, aggression, confusion, and psychosis.
Side Effects
The most common side effects of donepezil are listed below:
Nausea
Diarrhea
Trouble sleeping
Vomiting
Muscle cramps
Tiredness
Decreased appetite
Metabolism
Donepezil is metabolized by first pass metabolism in the liver, primarily by CYP3A4, in addition to CYP2D6. After this, O-dealkylation, hydroxylation, N-oxidation, hydrolysis, and O-glucuronidation occur, producing various metabolites with similar half-lives to the unchanged parent drug. A study of the pharmacokinetics of radiolabeled donepezil demonstrated that about 53% of plasma radioactivity appeared as donepezil in the unchanged form, and 11% was identified as the metabolite 6-O-desmethyl donepezil, which exerts similar potency inhibition of the acetylcholinesterase enzyme. This drug is heavily metabolized to four primary metabolites, two of which are considered pharmacologically active, as well as to multiple inactive and unidentified metabolites.
Metabolism
When compared to tacrine, donepezil exhibits greater CNS AChE selectivity, longer elimination half-life (70–104 hours in subjects older than 55 years) and little or no potential for hepatotoxicity. Donepezil is metabolized by CYP2D6 and CYP3A4 via demethylation, debenzylation, hydroxylation, oxidation to the cis-N-oxide, and glucuronidation. The 6-O-desmethyl metabolite accounts for 11% of a dose, and it exhibits AChE inhibitory activity comparable to that of the parent compound.
Properties of Donepezil
| Melting point: | 207°C |
| Boiling point: | 527.9±50.0 °C(Predicted) |
| Density | 1.141±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Acetone (Slightly), Chloroform (Slightly) |
| form | Solid |
| pka | 8.84±0.10(Predicted) |
| color | White to Off-White |
| Water Solubility | 2.931 mg/L |
| CAS DataBase Reference | 120014-06-4(CAS DataBase Reference) |
Safety information for Donepezil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Donepezil
Donepezil manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Donepezil HCL / Base 99%View Details
Donepezil HCL / Base 99%View Details -
 Donepezil 98%View Details
Donepezil 98%View Details -
 120014-06-4 Donepezil 98%View Details
120014-06-4 Donepezil 98%View Details
120014-06-4 -
 Donepezil 120014-06-4 98%View Details
Donepezil 120014-06-4 98%View Details
120014-06-4 -
 120014-06-4 98%View Details
120014-06-4 98%View Details
120014-06-4 -
 Donepezil 99%View Details
Donepezil 99%View Details
120014-06-4 -
 Donepezil 99%View Details
Donepezil 99%View Details -
 Donepezil 98% CAS 120014-06-4View Details
Donepezil 98% CAS 120014-06-4View Details
120014-06-4
