Oxybutynin
- CAS NO.:5633-20-5
- Empirical Formula: C22H31NO3
- Molecular Weight: 357.49
- MDL number: MFCD00865252
- EINECS: 630-332-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
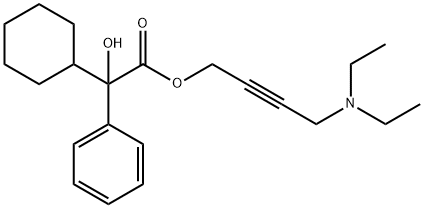
What is Oxybutynin?
Absorption
Oxybutynin should be swallowed whole with the help of liquids. A pharmacokinetic study revealed that oxybutynin was rapidly absorbed, and peak concentrations were reached within about 1 hour of administration, measured at 8.2 ngml-1 and AUC was 16 ngml-1. The biovailability of oxybutynin is about 6%, and the plasma concentration of the active metabolite, desethyloxybutynin is 5 to 12 times greater than that of oxybutynin. Bioavailability is increased in the elderly. Food has been shown to increase the exposure to controlled-release oxybutynin.
Toxicity
The acute oral LD50 in rats is 460 mg/kg.
Overdose information
An overdose with oxybutynin may manifest clinically as CNS overactivity, fever, palpitations, cardiac arrhythmias, urinary retention, respiratory failure, paralysis, in addition to coma. Provide supportive care immediately. Activated charcoal in addition to a cathartic agent should be administered. There have been 2 reports of an overdose with a 100 mg dose of oxybutynin. One case was a 13-year-old boy and another was a 34-year-old woman. Alcohol was also ingested simultaneously in both cases. The patients received supportive treatment and fully recovered.
Originator
Ditropan, Marion , US ,1975
The Uses of Oxybutynin
Oxybutynin(Ditropan) is an anticholinergic medication used to relieve urinary and bladder difficulties, including frequent urination and inability to control urination (urge incontinence), by decreasing muscle spasms of the bladder.Oxybutynin contains one
The Uses of Oxybutynin
Oxybutynin can be used as a pharmaceutical formulation for inhibitin body malodour.
The Uses of Oxybutynin
Oxybutynin is intended for relieving unpleasant symptoms associated with emptying the intestine or urinary bladder. A
Background
Overactive bladder (OAB) is a common condition negatively impacting the lives of millions of patients worldwide. Due to its urinary symptoms that include nocturia, urgency, and frequency, this condition causes social embarrassment and a poor quality of life.
Oxybutynin, also marketed as Ditropan XL, is an anticholinergic medication used for the relief of overactive bladder symptoms that has been optimized for high levels of safety and efficacy since initial FDA approval in 1975. This drug relieves undesirable urinary symptoms, increasing the quality of life for patients affected by OAB. It is often used as first-line therapy for OAB.
Indications
Oxybutynin is indicated for the symptomatic treatment of overactive bladder, which causes urge urinary incontinence and frequency, and urgency. Oxybutynin may also be used for children aged 6 and above for the symptomatic management of detrusor muscle overactivity which has been found to be related to a neurological condition. Spina bifida is an example of a neurological condition in which oxybutynin may be used to control urinary symptoms. On occasion, oxybutynin may be used off-label to relieve bladder spasms associated with ureteral stents or urinary catheters.
Definition
ChEBI: Oxybutynin is a racemate comprising equimolar amounts of (R)-oxybutynin and esoxybutynin. An antispasmodic used for the treatment of overactive bladder. It has a role as a muscarinic antagonist, a muscle relaxant, an antispasmodic drug, a parasympatholytic, a calcium channel blocker and a local anaesthetic. It is a tertiary amino compound and a racemate. It contains an esoxybutynin and a (R)-oxybutynin.
Manufacturing Process
A mixture of 394.2 grams of methyl phenylcyclohexylglycolate and 293.1 grams of 4-diethylamino-2-butynyl acetate was dissolved with warming in 2.6 liters of n-heptane. The solution was heated with stirring to a temperature of 60° to 70°C and 8.0 grams of sodium methoxide were added. The temperature of the mixture was then raised until the solvent began to distill. Distillation was continued at a gradual rate and aliquots of the distillate were successively collected and analyzed for the presence of methyl acetate by measurement of the refractive index. The reaction was completed when methyl acetate no longer distilled, and the refractive index observed was that of pure heptane (nD26 = 1.3855). About 3? hours were required for the reaction to be completed.
The reaction mixture was then allowed to cool to room temperature, washed with water, and extracted with four 165 ml portions of 2 N hydrochloric acid. The aqueous extracts were combined and stirred at room temperature to permit crystallization of the hydrochloride salt of the desired product. Crystallization was completed by cooling the slurry in an ice bath, and the product was collected by filtration, pressed dry, and recrystallized from 750 ml of water. Yield of pure crystalline material, 323 grams.
brand name
Oxytrol (Watson).
Therapeutic Function
Spasmolytic
General Description
Oxybutynin, 4-Diethylaminobut- 2-ynyl2-cyclohexyl-2- hydroxy-2-phenyl-ethanoate, (Oxytrol) wasone of the first agents specifically developed to exploit the effectsthat cholinergic blocking agents have on the bladder. Bycompetitively blocking the muscarinic receptors, it has directspasmolytic effects on bladder smooth muscle. This reductionin smooth muscle tone allows for greater volumes of urine tobe stored in the bladder, which results in less urinary incontinence,urgency, and frequency. Oxybutynin acts as a competitiveantagonist on M1, M2, and M3 receptor subtypes.
Pharmacokinetics
Oxybutynin exerts antispasmodic actions on the bladder, relieving the uncomfortable symptoms of overactive bladder, including urinary urgency and frequency. These actions occur through the inhibition of muscarinic receptors.
A note on angioedema and anticholinergic effects
Symptoms of angioedema may occur with oxybutynin therapy. If angioedema is suspected, discontinue oxybutynin immediately and provide appropriate medical treatment. In addition, anticholinergic effects may occur with the administration of this drug. Some symptoms may include hallucinations, confusion, agitation, and drowsiness. It is advisable to avoid operating heavy machinery before the response to oxybutynin has been monitored. Dose adjustments may be required.
Synthesis
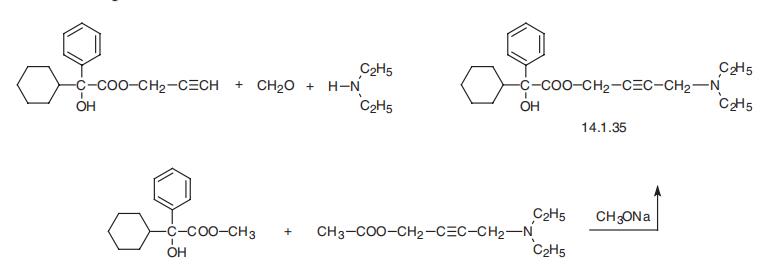
Oxybutynin, 4-diethylamino-2-butynylic ester |á-phenylclohexaneglycolic acid (14.1.35), is synthesized either by a Mannich reaction using propargyl ester of |á-phenyl-|á-cyclohexaneglycolic acid, paraform and diethylamine, or transesterification of the methyl ester of |á-phenyl-|á-cyclohexaneglycolic acid using 1-acetoxy-4-diethylamino- 2-butene in the presence of sodium methoxide [26].
Veterinary Drugs and Treatments
Oxybutynin may be useful for the adjunctive therapy of detrusor hyperreflexi
Metabolism
Oxybutynin is heavily metabolized by the CYP3A4 enzyme system in both the liver and the wall of the intestine. It undergoes first-pass metabolism, and its resulting primary active metabolite, N-desethyloxybutynin circulates. It is active at the muscarinic receptors in both the bladder and the salivary gland. Hepatic biotransformation also produces its major inactive metabolite, phenylcyclohexylglycolic acid.
Properties of Oxybutynin
| Boiling point: | 494.4±45.0 °C(Predicted) |
| Density | 1.097±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | ≥ 17.9mg/mL in DMSO |
| pka | 8.04(at 25℃) |
| form | Powder |
| color | White to off-white |
| CAS DataBase Reference | 5633-20-5(CAS DataBase Reference) |
Safety information for Oxybutynin
Computed Descriptors for Oxybutynin
Oxybutynin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 5633-20-5 Oxybutynin 98%View Details
5633-20-5 Oxybutynin 98%View Details
5633-20-5 -
 5633-20-5 98%View Details
5633-20-5 98%View Details
5633-20-5 -
 Oxybutynin 98%View Details
Oxybutynin 98%View Details
5633-20-5 -
 Oxybutynin 5633-20-5 98%View Details
Oxybutynin 5633-20-5 98%View Details
5633-20-5 -
 5633-20-5 Oxybutynin 98%View Details
5633-20-5 Oxybutynin 98%View Details
5633-20-5 -
 Oxybutynin 98% CAS 5633-20-5View Details
Oxybutynin 98% CAS 5633-20-5View Details
5633-20-5 -
 Oxybutynin 98% (HPLC) CAS 5633-20-5View Details
Oxybutynin 98% (HPLC) CAS 5633-20-5View Details
5633-20-5 -
 Oxybutynin 5 Mg TabView Details
Oxybutynin 5 Mg TabView Details
5633-20-5
