Midodrine hydrochloride
Synonym(s):(±)-1-(2,5-Dimethoxyphenyl)-2-glycinamidoethanol;Midodrine hydrochloride
- CAS NO.:3092-17-9
- Empirical Formula: C12H19ClN2O4
- Molecular Weight: 290.74
- MDL number: MFCD00079455
- EINECS: 622-590-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
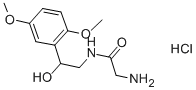
What is Midodrine hydrochloride?
Chemical properties
Crystalline Solid
Originator
Gutron,Hormonchemie,W. Germany,1977
The Uses of Midodrine hydrochloride
A phenylalkanolamine derivative which has been found to be effective in treating hypertensive conditions due to their long lasting blood pressure increasing effects
The Uses of Midodrine hydrochloride
morphine antagonist
What are the applications of Application
Midodrine, Hydrochloride is a phenylalkanolamine derivative which has been found to increase arterial pressure
Definition
ChEBI: A hydrochloride resulting from the combination of equimolar amounts of midodrine and hydrogen chloride. Midodrine is a direct-acting sympathomimetic with selective alpha-adrenergic agonist activity. The hydrochloride salt is used as a periph ral vasoconstrictor in the treatment of certain hypotensive states. The main active moiety is its major metabolite, deglymidodrine.
Manufacturing Process
19.5 parts of carbobenzoxyglycine, 7.1 parts of triethylamine and 162 parts ofdry toluene are mixed with 11.2 parts of isovaleric acid chloride at 0°C toform the mixed anhydride and the mixture is agitated for two hours at 0°C.32.4 parts of 1-(2',5'-dimethoxyphenyl)-2-aminoethanol-(1) are then added,the mixture is agitated for four hours at a temperature between 0°C and+10°C and then left to stand overnight at that temperature. A thick crystalpaste forms. The reaction product is dissolved in 450 parts of ethyl acetateand 200 parts of water. The ethyl acetate solution is separated, washed withhydrochloric acid, sodium bicarbonate solution and water, dried over sodiumsulfate and inspissated. The inspissation residue is digested with 342 parts ofxylene, the required product crystallizing out. 34.9 parts of 1-(2',5'-dimethoxyphenyl)-2-(N-carbobenzoxyglycineamido)-ethanol-(1) are obtained.66.2 parts of 1-(2',5'-dimethoxyphenyl)-2-(N-carbobenzoxyglycineamido)-ethanol-(1) are hydrogenated in the presence of 6.6 parts of palladium carbon(10%) in 2,000 parts of glacial acetic acid. When no more hydrogen isabsorbed (3 mols of hydrogen are used), hydrogenation stops. The catalyst isremoved by suction and the equivalent quantity of hydrochloric acid in ethanolis added to the filtrate with agitation. During further agitation at roomtemperature 28.6 parts of crude 1-(2',5'-dimethoxyphenyl)-2-glycineamidoethanol-(1)hydrochloride crystallize, and are isolated andrecrystallized from water-methanol for purification. 22.1 parts of pure productare obtained with a melting point of 192°C to 193°C.
An alternative synthesis route is described by Kleeman and Engel.
brand name
Orvaten (UpsherSmith); Proamatine (Shire).
Therapeutic Function
Peripheral vasotonic, Antihypotensive
General Description
Midodrine is the N-glycyl prodrug ofthe selectiveα1-agonist desglymidodrine. Removal of theN-glycyl moiety from midodrine occurs readily in the liver aswell as throughout the body, presumably by amidases.Midodrine is orally active and represents another example ofa dimethoxy-β-phenylethylamine derivative that is used therapeuticallyfor its vasoconstrictor properties. Specifically, it isused in the treatment of symptomatic orthostatic hypotension.
Clinical Use
Treatment of orthostatic hypotension, including dialysis related hypotension
Drug interactions
Potentially hazardous interactions with other drugs
Adrenergic neurone blockers: midodrine antagonises
hypotensive effect.
Anaesthetics: risk of arrhythmias if given with
volatile anaesthetics.
Antidepressants: risk of arrhythmias and
hypertension if given with tricyclic antidepressants,
MAOIs and moclobemide.
Antihypertensives: antagonise hypertensive effect of
midodrine; risk of severe hypertension if given with
beta-blockers.
Dopaminergics: avoid with rasagiline and selegiline.
Other drugs which increase blood pressure:
enhanced hypertensive effect.
Metabolism
Undergoes enzymatic hydrolysis in the systemic circulation to an active metabolite (desglymidodrine)
Properties of Midodrine hydrochloride
| Melting point: | 192-193°C |
| storage temp. | -20°C Freezer |
| form | neat |
| CAS DataBase Reference | 3092-17-9(CAS DataBase Reference) |
Safety information for Midodrine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Midodrine hydrochloride
| InChIKey | MGCQZNBCJBRZDT-UHFFFAOYSA-N |
Midodrine hydrochloride manufacturer
Enaltec Labs Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Midodrine 99%View Details
Midodrine 99%View Details -
 3092-17-9 98%View Details
3092-17-9 98%View Details
3092-17-9 -
 Midodrine hydrochloride 99%View Details
Midodrine hydrochloride 99%View Details
3092-17-9 -
 Midodrine hydrochloride CAS 3092-17-9View Details
Midodrine hydrochloride CAS 3092-17-9View Details
3092-17-9 -
 Midodrine hydrochloride CAS 3092-17-9View Details
Midodrine hydrochloride CAS 3092-17-9View Details
3092-17-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1