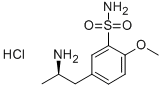
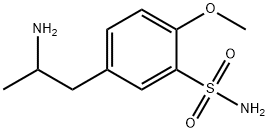
Tamsulosin
- CAS NO.:106133-20-4
- Empirical Formula: C20H28N2O5S
- Molecular Weight: 408.51
- MDL number: MFCD00871531
- EINECS: 600-716-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:17:46
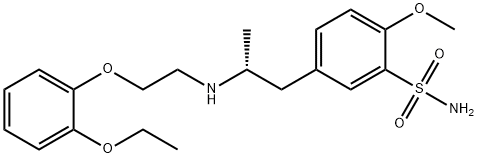
What is Tamsulosin?
Absorption
Oral tamsulosin is 90% absorbed in fasted patients. The area under the curve is 151-199ng/mL*hr for a 0.4mg oral dose and 440-557ng/mL*hr for a 0.8mg oral dose. The maximum plasma concentration is 3.1-5.3ng/mL for a 0.4mg oral dose and 2.5-3.6ng/mL for a 0.8mg oral dose. Taking tamsulosin with food increases the time to maximum concentration from 4-5 hours to 6-7 hours but increases bioavailability by 30% and maximum plasma concentration by 40-70%.
Toxicity
In the event of overdose, patients may experience hypotension and should lie down in a supine position to maintain blood pressure and heart rate. If further measures are required intravenous fluids should be considered. If further progression is required, vasopressors may be used and renal function should be monitored. Dialysis is unlikely to assist in treating overdose because tamsulosin is extensively protein bound.
The oral LD50 in rats is 650mg/kg.
Tamsulosin is not indicated for use in women and no studies have been performed in pregnancy, though animal studies have not shown fetal harm. Tamsulosin is excreted in the milk of rats but there is no available data on what the effect of this tamsulosin exposure may be. Animal studies have shown male and female rat fertility is affected by tamsulosin due to impairment of ejaculation and fertilization. In men, tamsulosin is associated with abnormal ejaculation. Tamsulosin is not mutagenic but may be carcinogenic at levels above the maximum recommended human dose. Female rats experience a slight increase in the rates of mammary gland fibroadenomas and adenocarcinomas.
Description
Tamsulosin hydrochloride is the first in the class of potent and selective α- 1A adrenoceptor antagonists introduced for the treatment of dysuria associated with benign prostatic hypertrophy (BPH). In a clinical study, significant improvement in irritative and obstructive symptoms has been reported for tamsulosin treated patients with BPH. ln vitro studies in human penile erectile tissue and vas deferens indicated that tamsulosin may be of use in treating male sexual dysfunction.
Originator
Yamanouchi (Japan)
The Uses of Tamsulosin
tamsulosin
The Uses of Tamsulosin
chemotherapy drug
Indications
Tamsulosin is indicated for the treatment of signs and symptoms of benign prostatic hyperplasia.
Tamsulosin is also used off label for the treatment of ureteral stones, prostatitis, and female voiding dysfunction.
Background
Tamsulosin is a selective alpha-1A and alpha-1B adrenoceptor antagonist that exerts its greatest effect in the prostate and bladder, where these receptors are most common. It is indicated for the treatment of signs and symptoms of benign prostatic hypertrophy. Antagonism of these receptors leads to relaxation of smooth muscle in the prostate and detrusor muscles in the bladder, allowing for better urinary flow. Other alpha-1 adrenoceptor antagonists developed in the 1980s were less selective and more likely to act on the smooth muscle of blood vessels, resulting in hypotension.
Tamsulosin was first approved by the FDA on April 15, 1997.
Definition
ChEBI: Tamsulosin is a 5-(2-{[2-(2-ethoxyphenoxy)ethyl]amino}propyl)-2-methoxybenzenesulfonamide that has (R)-configuration. A specific alpha1 adrenoceptor antagonist used (generally as its hydrochloride salt, tamsulosin hydrochloride) in the treatment of prostatic hyperplasia, chronic prostatitis, urinary retention, and help with the passage of kidney stones. It has a role as an alpha-adrenergic antagonist and an antineoplastic agent. It is a conjugate base of a tamsulosin(1+). It is an enantiomer of an ent-tamsulosin.
Manufacturing Process
In 1,000 ml of acetonitrile was suspended 17 g of 5-{2-[2-(2-
ethoxyphenoxy)ethylamino]-1-hydroxy-2-methylethyl}-2-
methoxybenzenesulfonamide hydrochloride and while stirring the suspension,
9 g of thionyl chloride was added dropwise to the suspension at room
temperature, whereby the product first dissolved and then began to crystallize
gradually. After stirring the mixture for two days, the crystals formed were
recovered by filtration, washed with chloroform and dried to provide 15 g of
5-{1-chloro-2-[2-(2-ethoxyphenoxy)ethylamino]-2-methylethyl}-2-
methoxybenzenesulfonamide hydrochloride. Melting point: 197°-200°C.
In methanol was dissolved the 5-{1-chloro-2-[2-(2-
ethoxyphenoxy)ethylamino]ethyl}-2-methoxybenzenesulfonamide
hydrochloride and after adding thereto 10% palladium carbon, dechlorination
was performed under hydrogen stream at normal temperature and pressure.
The palladium carbon was filtered away and the filtrate was concentrated
under reduced pressure to provide the 2-methoxy-5-{2-[2-(2-
ethoxyphenoxy)ethylamino]ethyl}benzenesulfonamide hydrochloride, which
was recrystallized from 120 ml of a mixture of methanol and ethanol (1:4 by
volume ratio) to provide the colorless crystals thereof. The melting point of
the 5-{2-[2-(2-ethoxyphenoxy)ethylamino]-2-methylethyl}-2-
methoxybenzenesulfonamide hydrochloride: 254°-256°C.
brand name
Harnal
Therapeutic Function
Antihypertensive
General Description
Tamsulosin (Flomax), a nonquinazoline benzensulfonamide,is the first in the class of subtype selective 1Ablocker.It is many folds more selective for α1A-receptorsthan for the other α1-receptors. This selectivity may favorblockade of α1A-receptors found in the prostate gland overthose found in vascular tissue. Tamsulosin is efficacious inthe treatment of BPH with little effect on blood pressure.Orthostatic hypotension is not as great with this agent aswith the nonselective quinazolines.
Pharmacokinetics
Tamsulosin is an alpha adrenoceptor blocker with specificity for the alpha-1A and alpha-1D subtypes, which are more common in the prostate and submaxillary tissue. The final subtype, alpha-1B, are most common in the aorta and spleen. Tamsulosin binds to alpha-1A receptors 3.9-38 times more selectively than alpha-1B and 3-20 times more selectively than alpha-1D. This selectivity allows for a significant effect on urinary flow with a reduced incidence of adverse reactions like orthostatic hypotension.
Metabolism
Tamsulosin is mostly metabolized in the liver by cytochrome P450 (CYP) 3A4 and 2D6, with some metabolism by other CYPs. CYP3A4 is responsible for the deethylation of tamsulosin to the M-1 metabolite and the oxidative deamination to the AM-1 metabolite, while CYP2D6 is responsible for the hydroxylation of tamsulosin to the M-3 metabolite and the demethylation of tamsulosin to the M-4 metabolite. In addition, tamsulosin can be hydroxylated at a different position by an unknown enzyme to form the M-2 metabolite. The M-1, M-2, M-3, and M-4 metabolites can be glucuronidated, and the M-1 and M-3 metabolites can undergo sulfate conjugation to form other metabolites before excretion.
Properties of Tamsulosin
| Melting point: | 226-228°C |
| Boiling point: | 595.5±60.0 °C(Predicted) |
| Density | 1.191±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO: 100 mg/mL (244.79 mM) |
| pka | 10.08±0.60(Predicted) |
| form | Solid |
| color | White to off-white |
| CAS DataBase Reference | 106133-20-4(CAS DataBase Reference) |
Safety information for Tamsulosin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tamsulosin
Tamsulosin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



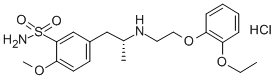
You may like
-
 106133-20-4 Tamsulosin Base 98%View Details
106133-20-4 Tamsulosin Base 98%View Details
106133-20-4 -
 106133-20-4 98%View Details
106133-20-4 98%View Details
106133-20-4 -
 TAMSULOSINE 98%View Details
TAMSULOSINE 98%View Details -
 Tamsulosin 98%View Details
Tamsulosin 98%View Details -
 Tamsulosin 98%View Details
Tamsulosin 98%View Details -
 Tamsulosin 98%View Details
Tamsulosin 98%View Details -
 Tamsulosin 95% CAS 106133-20-4View Details
Tamsulosin 95% CAS 106133-20-4View Details
106133-20-4 -
 Tamsulosin API PowderView Details
Tamsulosin API PowderView Details
106133-20-4
