Metandienone
Synonym(s):1-Dehydro-17α-methyltestosterone;Dianabol;Methandienone;Methandrostenolone
- CAS NO.:72-63-9
- Empirical Formula: C20H28O2
- Molecular Weight: 300.44
- MDL number: MFCD00003650
- EINECS: 200-787-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-11 16:58:07
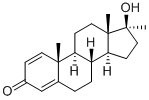
What is Metandienone?
Chemical properties
White Solid
Originator
Dianabol,Ciba,US,1960
The Uses of Metandienone
Anabolic steroid. Androgen. Controlled substance.
Definition
ChEBI: Methandrostenolone is an organic molecular entity.
Manufacturing Process
As described in US Patent 2,929,763, methandrostenolone may be made by a fermentation route. 2 g of sodium nitrate, 1 g of primary potassium orthophosphate, 0.5 g of magnesium sulfate heptahydrate, 0.5 g of potassium chloride, 50 g of glucose and 1 g of Difco yeast extract are dissolved in one liter of tap water, brought to pH 5 by addition of a sodium hydroxide solution and sterilized. The resulting nutrient solution is inoculated with 50 cc of a 4- day-old shaking culture of Didymella lycopersici and shaken for 48 hours at 27°C, whereby the culture becomes well developed.
To two liters of a culture so prepared there is added under sterile conditions a solution of 500 mg of 17α-methyl-testosterone in 15 cc of acetone. Shaking is carried out for 3 days at 27°C, the mycellium then filtered off with suction, washed with water and ethyl acetate and the combined filtrates extracted with ethyl acetate. The extraction residue obtained after evaporation of the solvent is dissolved in a little acetone. On addition of ether, the 1-dehydro-17αmethyl-testosterone is obtained in compact crystals. MP 163° to 164°C.
An alternative synthetic route is described in US Patent 2,900,398 as follows. A suspension of 30 g of 17α-methyl-testosterone and 10 g of selenium dioxide in 600 cc of tertiary amyl alcohol is treated with 60 g of magnesium powder and 6 cc of glacial acetic acid.
The mixture is refluxed for 24 hours with good stirring in an atmosphere of nitrogen, another 10 g of selenium dioxide being added after 10 hours. After some cooling, the suspension is filtered through some Hyflo and washed thoroughly with ethyl acetate. The resulting brown solution is evaporated in vacuo and the residue dissolved in ethyl acetate.
The ethyl acetate solution is then washed with water, dried and evaporated. To remove any selenium still present, the residue is dissolved in 200 cc of methanol and mixed with 100 g of iron powder and 2 g of active carbon. The mixture is heated for 30 minutes with stirring under reflux, then filtered with suction, washed with methanol and the solution evaporated in vacuo. The residue is then chromatographed on 900 g of aluminum oxide. The residues of the evaporated benzene and ether fractions are treated with active carbon in methanol or acetone, evaporated again, and the residue recrystallized from a mixture of acetone and ether. There are obtained 17.5 g of pure 1-dehydro17α-methyl-testosterone which melts at 163° to 164°C.
Therapeutic Function
Androgen, Anabolic
Properties of Metandienone
| Melting point: | 165-166 °C |
| Boiling point: | 381.66°C (rough estimate) |
| Density | 1.0597 (rough estimate) |
| refractive index | 1.4800 (estimate) |
| Flash point: | -2℃ |
| storage temp. | 2-8°C |
| solubility | Acetonitrile: 1 mg/ml; Ethanol: 1 mg/ml; Methanol: 1 mg/ml |
| pka | 15.12±0.60(Predicted) |
| form | neat |
| optical activity | [α]1.15/D 0.5±0.5° in chloroform |
| Merck | 5951 |
| BRN | 2056255 |
| InChI | InChI=1/C20H28O2/c1-18-9-6-14(21)12-13(18)4-5-15-16(18)7-10-19(2)17(15)8-11-20(19,3)22/h6,9,12,15-17,22H,4-5,7-8,10-11H2,1-3H3/t15-,16+,17+,18+,19+,20+/s3 |
| CAS DataBase Reference | 72-63-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Methandrostenolone(72-63-9) |
Safety information for Metandienone
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Metandienone
| InChIKey | XWALNWXLMVGSFR-HLXURNFRSA-N |
| SMILES | [C@]12(CCC3=CC(=O)C=C[C@]3(C)[C@]1(CC[C@]1(C)[C@](O)(C)CC[C@@]21[H])[H])[H] |&1:0,9,11,14,16,21,r| |
Metandienone manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Methandienone solution CAS 72-63-9View Details
Methandienone solution CAS 72-63-9View Details
72-63-9 -
 17β-Hydroxy-17-methylandrosta-1,4-dien-3-one CAS 72-63-9View Details
17β-Hydroxy-17-methylandrosta-1,4-dien-3-one CAS 72-63-9View Details
72-63-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1