Oxandrolone
Synonym(s):17β-Hydroxy-17α-methyl-2-oxa-5α-androstan-3-one;Oxandren;Provitar;Vasorome
- CAS NO.:53-39-4
- Empirical Formula: C19H30O3
- Molecular Weight: 306.44
- MDL number: MFCD00198944
- EINECS: 200-172-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-30 16:33:02

What is Oxandrolone?
Toxicity
The oral LD50 of oxandrolone in mice and dogs is greater than 5,000 mg/kg.
Description
Oxandrolone is a synthetic anabolic-androgenic steroid. It is a 17 alpha-methylated version of dihydrotestosterone (DHT). It can be used for the treatment of many kinds of disorders such as idiopathic short stature, body mass loss due to catabolic illness, severe burns, trauma and hereditary angioedema as well as turner syndrome. Oxandrolone is especially effective in the treatment of severe burns without causing obvious side effects. It acts as an agonist of the androgen receptor, which modulates related gene expression to increase protein synthesis, further boosting muscle growth and increasing body mass as well as bone mineral density. However, it should be noted that its androgenic effect is less than its anabolic effect.
Chemical properties
white Solid. Soluble in chloroform, slightly soluble in ethanol, acetone, slightly soluble in ether, very slightly soluble in water.
Originator
Anavar,Searle,US,1964
The Uses of Oxandrolone
Oxandrolone is used for the same indications as nandrolone. It is a synthetic, anabolic steroid. Used to promote muscle growth and combat involuntary weight loss. It has also been used to treat cases of osteoporosis.
The Uses of Oxandrolone
androgenic anabolic steroid;reverses catabolic tissue processes; promotes buildup of protein; increases erythropoietin production
Background
A synthetic hormone with anabolic and androgenic properties.
Indications
Use to promote weight gain after weight loss following extensive surgery.
Definition
ChEBI: Oxandrolone is a 3-oxo steroid, an oxa-steroid, a 17beta-hydroxy steroid and an anabolic androgenic steroid. It has a role as an androgen and an anabolic agent.
brand name
Oxandrin (Savient).
Therapeutic Function
Androgen
General Description
Oxandrolone, 17β-hydroxy-17-methyl-2-oxaandrostan-3-one, is approved to aid in the promotionof weight gain after weight loss following surgery,chronic infections, or severe trauma and to offset protein catabolismassociated with long-term corticosteroid use.Oxandrolone is also used to relieve bone pain accompanyingosteoporosis. It has been used to treat alcoholic hepatitisand HIV wasting syndrome.
Biochem/physiol Actions
Oxandrolone is a synthetic anabolic steroid. It is is a non-aromatizable androgen with no estrogenic effects and with mild androgenic activity. Clinical uses of oxandrolone include to promote weight gain after weight loss following extensive surgery or chronic infections or trauma, to offset the protein catabolism associated with prolonged administration of corticosteroids, to relieve bone pain frequently accompanying osteoporosis, and to treat Turner′s syndrome in girls.
Pharmacokinetics
Oxandrolone is an anabolic steroids indicated as adjunctive therapy to promote weight gain after weight loss following extensive surgery, chronic infections, or severe trauma, and in some patients who without definite pathophysiologic reasons fail to gain or to maintain normal weight, to offset the protein catabolism associated with prolonged administration of corticosteroids, and for the relief of the bone pain frequently accompanying osteoporosis. Anabolic steroids are synthetic derivatives of testosterone.
Safety Profile
Moderately toxic by ingestion andintraperitoneal routes. Experimental reproductive effects.When heated to decomposition it emits acrid smoke andfumes.
Synthesis
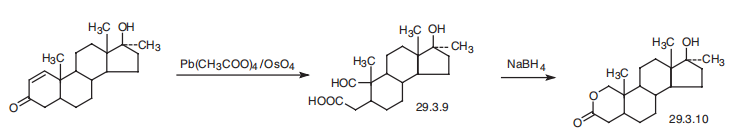
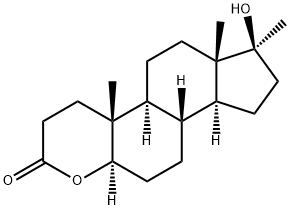
Oxandrolone, 17|?-hydroxy-17|á-methyl-2-oxa-5-androstan-3-one (29.3.10), is made by oxidation of the C1¨CC2 double bond of 17|?-hydroxy-17|á-methyl-1-androsten- 3-one by a mixture of lead tetraacetate and osmium tetroxide with an opening of the A ring of the steroid system, which forms an aldehyde acid (29.3.9). Upon reducing the aldehyde group with sodium borohydride, intramolecular cyclization takes place, directly forming a lactone (29.3.10), which is the desired oxandrolone.
Metabolism
Renal
References
Li, H., et al. "The efficacy and safety of oxandrolone treatment for patients with severe burns: A systematic review and meta-analysis. " Burns Journal of the International Society for Burn Injuries 42.4(2016): 717.
Hart, D. W., et al. "Anabolic effects of oxandrolone after severe burn." Annals of Surgery 233.4(2001): 556-564.
Sheffieldmoore, M, et al. "Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. " Journal of Clinical Endocrinology & Metabolism 84.8(1999): 2705-11.
https://en.wikipedia.org/wiki/Oxandrolone
Properties of Oxandrolone
| Melting point: | 235-238°C |
| Boiling point: | 387.04°C (rough estimate) |
| alpha | D25 -23° (c = approx 1% in chloroform) |
| Density | 1.0829 (rough estimate) |
| refractive index | 1.4200 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble2mg/mL (clear solution; warmed) |
| form | powder |
| pka | 15.15±0.60(Predicted) |
| color | white to beige |
| optical activity | [α]/D -19 to -26°, c = 1 (CDCl3) |
| Merck | 6921 |
| CAS DataBase Reference | 53-39-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Oxandrolone(53-39-4) |
Safety information for Oxandrolone
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Oxandrolone
| InChIKey | QSLJIVKCVHQPLV-PEMPUTJUSA-N |
Oxandrolone manufacturer
Omicron Pharmatech Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 Oxandrolone 99%View Details
Oxandrolone 99%View Details -
 Oxandrolone 99%View Details
Oxandrolone 99%View Details -
 Oxandrolone 98%View Details
Oxandrolone 98%View Details -
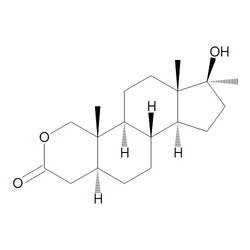 OXANDROLONE CAS 53-39-4View Details
OXANDROLONE CAS 53-39-4View Details
53-39-4 -
 Anavar Steroids Oxandrolone PowderView Details
Anavar Steroids Oxandrolone PowderView Details
53-39-4 -
 Oxandrolone API POWDERView Details
Oxandrolone API POWDERView Details
53-39-4 -
 Anavar Oxandrolone 10 Mg TablesView Details
Anavar Oxandrolone 10 Mg TablesView Details
53-39-4 -
 Oxandrolone 10 Mg TabletsView Details
Oxandrolone 10 Mg TabletsView Details
53-39-4
