Dehydroepiandrosterone
Synonym(s):DHEA;Dehydroepiandrosterone;Prasterone;Dehydroisoandrosterone;3β-Hydroxy-5-androsten-17-one
- CAS NO.:53-43-0
- Empirical Formula: C19H28O2
- Molecular Weight: 288.43
- MDL number: MFCD00003613
- EINECS: 200-175-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-28 12:01:23
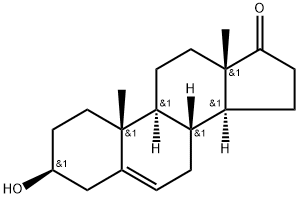
What is Dehydroepiandrosterone?
Absorption
Following a 50-mg DHEA PO dose in cynomolgus monkeys, systemic availability was only 3.1 +/- 0.4%. [PMID: 12970301]
Toxicity
Acute oral toxicity (LD50): >10000 mg/kg [Rat]. Lowest Published Toxic Dose (TDL) [Man] - Route: Oral; Dose: 10 mg/kg/2W intermittent.
Description
Dehydroepiandrosterone(DHEA) is an endogenous steroid hormone that is secreted primarily by the adrenal gland and is the most abundant sex steroid. It is a neurohormone; small quantities of DHEA are produced in the brain and declines in serum and CSF with age. (Knopman and Henderson, 2003). Metabolites of DHEA include androstenedione , which subsequently may be metabolized to testosterone or estrone which is an estradiol precursor. In addition to serving as an intermediate in the biosynthesis of sex steroids, DHEA directly modulates a number of cellular and nuclear receptors.
Chemical properties
white fine crystalline powder. There are two crystal forms. Needle-like crystal, melting point 140-141oC; small leaf-like crystal, melting point 152-153oC. Has right-handedness. Soluble in alcohol, ether, benzene, slightly soluble in chloroform, petroleum ether. In case of digitonin to produce precipitation.
Originator
Aslera ,Genelabs Technologies, Inc.
Occurrence
DHEA is naturally occurring in yam (see Wild Yam, p. 596-597).
The Uses of Dehydroepiandrosterone
Dehydroepiandrosterone (DHEA) is a major C19 steroid produced by the adrenal cortex. It is a major secretory steroidal product of the adrenal gland; secretion progresively declines with aging. May have estrogen-or androgen-like effects depending on the hormonal milieu. Intracellularly converted to androstenedione. It is used in treatment of menopausal syndrome. People living with HIV may be interested in taking DHEA for a variety of reasons, including treatment of depression, increasing bone density, decreasing arterial plaques, improvement of immune function with HIV, increased memory, and increased muscle strength.
Indications
DHEA is taken as a supplement for a variety of unsubstantiated indications. The following indications have shown promise and are backed up by some scientific evidence: schizophrenia (DHEA may be more effective in women than men); improving the appearance of older people’s skin (taking DHEA by mouth seems to increase skin thickness and moisture, and decrease facial “age spots” in elderly men and women); improving ability to achieve an erection in men with sexual dysfunction. Additionally, DHEA has shown promise in improving symptoms of lupus (SLE). Taking DHEA by mouth along with conventional treatment may help reduce the number of times symptoms flare up and may allow a reduction in the dose of prescription drugs needed. DHEA may also help SLE symptoms such as muscle ache and mouth ulcers. DHEA also seems to strengthen bones in SLE patients being treated with high-dose steroids (corticosteroids). DHEA also shows promise in the treatment of osteoporosis. Taking DHEA by mouth daily seems to improve bone mineral density (BMD) in older women and men with osteoporosis or osteopenia (pre-osteoporosis). DHEA may also increase BMD in young women with the eating disorder called anorexia nervosa. DHEA is often prescribed in India for the induction of ovulation to improve chances of pregnancy.
Background
Prasterone, also known as dehydroepiandrosterone (DHEA) is a major C19 steroid produced by the adrenal cortex. It is also produced in small quantities in the testis and the ovary. Dehydroepiandrosterone (DHEA) can be converted to testosterone; androstenedione; estradiol; and estrone. Most of DHEA is sulfated (dehydroepiandrosterone sulfate) before secretion.
In the United States, DHEA or DHEAS have been advertised with claims that they may be beneficial for a wide variety of ailments. DHEA and DHEAS are readily available in the United States, where they are marketed as over-the-counter dietary supplements. In November 2016, DHEA was approved (as Intrarosa) to treat women experiencing moderate to severe pain during sexual intercourse (dyspareunia), a symptom of vulvar and vaginal atrophy (VVA), due to menopause.
In Canada, a prescription is required to buy DHEA.
What are the applications of Application
DHEA is a negative modulator of GABAA receptors
Definition
ChEBI: Dehydroepiandrosterone is an androstanoid that is androst-5-ene substituted by a beta-hydroxy group at position 3 and an oxo group at position 17. It is a naturally occurring steroid hormone produced by the adrenal glands. It has a role as an androgen, a human metabolite and a mouse metabolite. It is a 17-oxo steroid, an androstanoid and a 3beta-hydroxy-Delta(5)-steroid.
Manufacturing Process
To a solution of 1 gram of 16-dehydropregnenolon-3β-acetate in 10 ml pyridine is added 0.22 gram of hydroxylamine hydrochloride, and the mixture is allowed to stand at room temperature for four days. One gram of 16dehydropregnenolon-3β-acetate oxime is dissolved in 30 ml of hot dioxane, and then the solution is cooled in an ice bath until about one-half of the dioxane has solidified. Then 1 gram of phosphorus pentachloride is added and the mixture is shaken until all the dioxane has melted. The mixture is maintained at 35°C, for seventy-five minutes, then an excess of ice is added and the solution is again allowed to stand at 35°C. After about thirty minutes, a solution of 5 ml of concentrated hydrochloric acid in 10 ml of water is added, and the mixture is diluted with water, extracted with ether and the ethereal extract washed with dilute sodium hydroxide solution. The ether is removed on a steam bath and the residue is worked up to yield dehydroepiandrosterone.
brand name
17-chetovis 17-hormoforin;Cetavister;Climatost;Dastonil;Dha-s (prasterone);Gynodian;Longevital 5000;Maxepa;Mentalormon;Mylis;Neurocotex;Psicosterone;Ro 66827;Sh 833;Ultrapla.
Therapeutic Function
Glucocorticoid
World Health Organization (WHO)
The World Health Organization has no information further to the above regarding preparations containing prasterone or to indicate that such preparations remain available.
General Description
DHEA is a major secretory steroidal product of the adrenal gland and is a hormonal precursor to both androgens and estrogens. It can also be synthesized using wild yam or soy, but there is no evidence to show that humans are able to increase DHEA levels by consuming these foods. DHEA and its sulfated metabolite, DHEA-S, are negative modulators of GABAA receptors.
Health Hazard
An experimental teratogen.Experimental reproductive effects. Questionablecarcinogen with experimental neoplastigenic data. Whenheated to decomposition it emits acrid smoke andirritating fumes.
Biochem/physiol Actions
Product does not compete with ATP.
Pharmacokinetics
DHEA is naturally produced from cholesterol through two cytochrome P450 enzymes. Cholesterol is converted to pregnenolone by the enzyme P450 scc (side chain cleavage); then another enzyme, CYP17A1, converts pregnenolone to 17α-Hydroxypregnenolone and then to DHEA. DHEA is increased by exercise and calorie restriction. Some theorize that the increase in endogenous DHEA brought about by calorie restriction is partially responsible for the longer life expectancy known to be associated with calorie restriction.
Side Effects
Side effects may include acne, skin rash, GI upset, hirsuitism, hypertension, and increased HDL. In people with HIV, additional side effects may include fatigue, nasal congestion, and headaches.
Safety
Dehydroepiandrosterone should always be used under the supervision of a medical professional. It is likely safe for people with low DHEA levels to take oral supplements short-term (<6 months) to restore DHEA to normal, but long-term use and doses resulting in high DHEA levels are possibly unsafe. Side effects are often seen with higher doses and longterm use.
Metabolism
Hepatic. As shown by their high conversion ratios (in a study involving cynomolgus monkeys), the major circulating metabolites of DHEA are DHEA-S, androsterone glucuronide, and androstane-3 alpha,17 beta-diol-glucuronide. [PMID: 12970301]
References
[1]. kimonides vg, khatibi nh, svendsen cn, et al. dehydroepiandrosterone (dhea) and dhea-sulfate (dheas) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. proc natl acad sci u s a, 1998, 95(4): 1852-1857.
[2]. suzuki m, wright ls, marwah p, et al. mitotic and neurogenic effects of dehydroepiandrosterone (dhea) on human neural stem cell cultures derived from the fetal cortex. proc natl acad sci u s a, 2004, 101(9): 3202-3207.
[3]. charalampopoulos i, tsatsanis c, dermitzaki e, et al. dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic bcl-2 proteins. proc natl acad sci u s a, 2004, 101(21): 8209-8214.
Properties of Dehydroepiandrosterone
| Melting point: | 149-151 °C(lit.) |
| Boiling point: | 370.65°C (rough estimate) |
| alpha | 12 º (c=2, ethanol 96% 25 ºC) |
| Density | 1.0484 (rough estimate) |
| refractive index | 1.4709 (estimate) |
| Flash point: | 9℃ |
| storage temp. | Hormones |
| solubility | insoluble in H2O; ≥13.7 mg/mL in DMSO; ≥58.6 mg/mL in ETOH |
| form | Fine Crystalline Powder |
| pka | 15.02±0.60(Predicted) |
| color | White |
| Water Solubility | 21.8mg/L(23.5 ºC) |
| Merck | 2871 |
| BRN | 2058110 |
| CAS DataBase Reference | 53-43-0 |
| NIST Chemistry Reference | 5-Androstene-3«beta»-ol-17-one(53-43-0) |
| EPA Substance Registry System | Prasterone (53-43-0) |
Safety information for Dehydroepiandrosterone
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Dehydroepiandrosterone
| InChIKey | FMGSKLZLMKYGDP-USOAJAOKSA-N |
Dehydroepiandrosterone manufacturer
AKASH PHARMA EXPORTS
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
![Dehydroepiandrosterone-[D6] sulfate sodium salt](https://img.chemicalbook.in/CAS/20211123/GIF/1261254-41-4.gif)


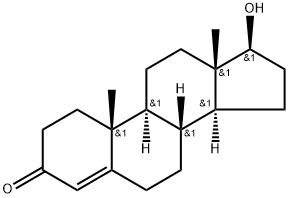
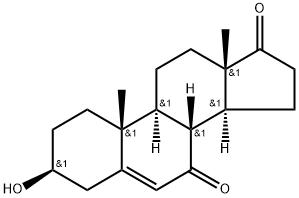
You may like
-
 Dehydroepiandrosterone 99%View Details
Dehydroepiandrosterone 99%View Details -
 Dehydro Epiandrosterone 99%View Details
Dehydro Epiandrosterone 99%View Details -
 DHEA 95.00% CAS 53-43-0View Details
DHEA 95.00% CAS 53-43-0View Details
53-43-0 -
 DHEA (trans-Dehydroandrosterone) 99% (HPLC) CAS 53-43-0View Details
DHEA (trans-Dehydroandrosterone) 99% (HPLC) CAS 53-43-0View Details
53-43-0 -
 Dehydroepiandrosterone CAS 53-43-0View Details
Dehydroepiandrosterone CAS 53-43-0View Details
53-43-0 -
 DEHYDRO-EPI-ANDROSTERONE For Biochemistry CAS 53-43-0View Details
DEHYDRO-EPI-ANDROSTERONE For Biochemistry CAS 53-43-0View Details
53-43-0 -
 Dehydroepiandrosterone 97% CAS 53-43-0View Details
Dehydroepiandrosterone 97% CAS 53-43-0View Details
53-43-0 -
 Dehydroepiandrosterone (DHEA) solution CAS 53-43-0View Details
Dehydroepiandrosterone (DHEA) solution CAS 53-43-0View Details
53-43-0
