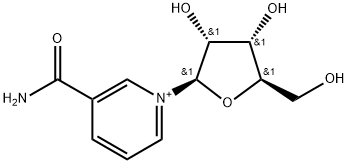
β-Nicotinamide Mononucleotide
Synonym(s):β-Nicotinamide ribose monophosphate;β-NMN;Nicotinamide ribotide;Nicotinamide-1-ium-1-β-D -ribofuranoside 5′-phosphate;NMN
- CAS NO.:1094-61-7
- Empirical Formula: C11H15N2O8P
- Molecular Weight: 334.22
- MDL number: MFCD00038748
- EINECS: 214-136-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-24 15:44:27
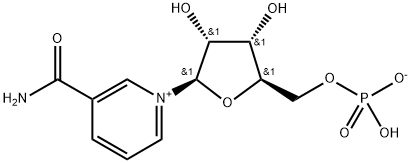
What is β-Nicotinamide Mononucleotide?
Description
Nicotinamide mononucleotide (NMN), a product of the NAMPT reaction and a key NAD+ intermediate, ameliorates glucose intolerance by restoring NAD+ levels in HFD-induced T2D mice. NMN also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation. NMN is used for studying binding motifs within RNA aptamers and ribozyme activation processes involving β-nicotinamide mononucleotide (β-NMN)-activated RNA fragments.
β-Nicotinamide mononucleotide (β-NMN) is an intermediate in the nicotinamide phosphoribosyltransferase (NAMPT)-catalyzed biosynthesis of nicotinamide adenine dinucleotide (NAD+). NAMPT mediates the condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate to produce β-NMN. β-NMN adenyltransferase subsequently converts β-NMN to NAD+.
Chemical properties
White to Yellowish lyophilized powder
The Uses of β-Nicotinamide Mononucleotide
β-Nicotinamide mononucleotide (NMN) is used to study binding motifs within RNA aptamers and ribozyme activation processes involving β-nicotinamide mononucleotide (β-NMN)-activated RNA fragments. NMN is a nucleotide derived from ribose and nicotinamide. Niacinamide (nicotinamide,) is a derivative of vitamin B3, also known as niacin.) As a biochemical precursor of NAD+, it may be useful in the prevention of pellagra.
β-Nicotinamide mononucleotide is an intermediate in the biosynthesis of nicotinamide adenine dinucleotide (NAD+). Nicotinamide phosphoribosyltransferase (Nampt) catalyzes the condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate to generate β-NMN, which is subsequently converted to NAD+ by β-NMN adenyltransferase.At 50-100 μM, β-NMN has been used to enhance NAD biosynthesis and glucose-stimulated insulin secretion in a Nampt+/- mouse model of metabolic disease, demonstrating a role for Nampt in β cell function.Furthermore, at 500 mg/kg/day, it has been shown to ameliorate glucose intolerance in high-fat diet-induced type 2 diabetes mice by restoring NAD+ levels.
What are the applications of Application
β-Nicotinamide mononucleotide is a product of the eNAMPT reaction and a NAD+ intermediate
Definition
ChEBI: β-Nicotinamide Mononucleotide is a condensation product of nicotinamide and ribose 5-phosphate, in which the nitrogen of nicotinamide is linked to the (β) c-l of the ribose. NMN zwitterion is a nicotinamide mononucleotide. It has a role as an Escherichia coli metabolite and a mouse metabolite. It is a conjugate base of a NMN(+). It is a conjugate acid of a NMN(-).
Preparation
β-Nicotinamide mononucleotide is a NAD+intermediate. In recent years, the relation of NAD+metabolism and aging-associated disease is attracting attention from various research fields.
Synthesis of β-nicotinamide mononucleotide (NMN)
A solution of NAD (3.5 g, 5.28 mmol) and ZrCl4(6.15 g, 26.4 mmol) in 500 ml water was stirred at 50°C for 30 min. The hydrolysis was monitored by TLC (SiO2EtOH/ 1 M NH4Ac [7 : 3]). The reaction was quenched with 245mL of a 0.5 M solution of Na3PO4. After adjusting to pH 7 with a 2 M solution of HCl, a white precipitate was formed. The suspension was centrifuged 8 min,1,000rpm, the supernatant was collected and the pellet was washed two times with 200 mL water. The combined supernatants wereconcentrated to 1/3 of its volume on a rotary evaporator. The remaining solution was purified with a column filled with Dowex 50WX8 (100-200 mesh, H+-Form, column-material: 2.5 x 30 cm). The column was loaded with 1.5 L5 % HCl and equilibrated with1.5L millipore water until pH 5 was reached. 100 mL of the concentrated solution was loaded on the ion exchange column and eluted with Milliporewater. The first cleavage product eluted was NMN (615 mg, 1.84 mmol,yield:35 %) and yielded a colorless solid after evaporation of the solvent, followed by AMP.
1H NMR (500MHz, D2O)δ: 9.48 (s, 1 H), 9.31 (d,J= 6.2 Hz, 1 H), 9.00 (d,J= 8.2 Hz, 1 H), 8.32 (dd,J= 8.2, 6.2 Hz, 1 H), 6.24 (d,J=5.4 Hz, 1 H), 4.68-4.64 (m, 1 H), 4.58 (t, 1 H), 4.48-4.45 (m, 1 H), 4.36–4.14 (m,J= 12.0, 2 H).
13C NMR (75 MHz, d2o) δ: 165.50, 145.65, 142.15, 139.53, 133.62, 128.19, 99.65, 87.18, 87.06, 77.42, 70.71, 63.89, 63.82.
31P NMR (202 MHz, D2O)δ:-0.03
What are the applications of Application
β-Nicotinamide mononucleotide (NMN) is a product of the extracellular Nicotinamide phosphoribosyltransferase (eNAMPT) reaction and a key NAD+ intermediate. It ameliorates glucose intolerance by restoring NAD+ levels in HFD-induced T2D mice . It also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation. It is used to study binding motifs within RNA aptamers and ribozyme activation processes involving β-nicotinamide mononucleotide (β-NMN)-activated RNA fragments.
Benefits
As one of the primary precursors of NAD+ and intermediaries in NAD+ biosynthesis, NMN is as essential as NAD+ in the body’s proper functioning of cells. NAD helps cells regulate a number of essential functions that help keep your cells running smoothly, including:energy metabolism, DNA repair, gene expression, and cellular stress responses.
General Description
β-Nicotinamide mononucleotide (β-NMN) is an intermediate in the nicotinamide phosphoribosyltransferase (NAMPT)-catalyzed biosynthesis of nicotinamide adenine dinucleotide (NAD+). NAMPT mediates the condensation of nicotinamide with 5-phosphoribosyl-1-pyrophosphate to produce β-NMN. β-NMN adenyltransferase subsequently converts β-NMN to NAD+.
Biochem/physiol Actions
In light of metabolic control mechanisms and many reports on β-Nicotinamide Mononucleotide (β-NMN), β-NMN is likely to be more effective as an NAD+ precursor than Nam. Research has compared the blood total NAD (NAD++NADH) and urinary excretion amounts of NAD+ catabolites in β-NMN- and Nam-administered rats. The concentration of blood total NAD and liver total NAD showed no significant differences among the three groups. However, when examining the kinetics of the urinary excretion, the urinary excretion of the SUM was lower in the β-NMN group than in the Nam group at 3-6 h after the administration. Moreover, the percentage of the urinary SUM was much lower in the β-NMN group than in the Nam group at 3-6 h. This result suggests that β-NMN is retained in the body for longer than Nam is. In addition, this result means that β-NMN has a higher turnover of salvage biosynthesis of NAD+ than Nam does. The resulting phenomenon accelerates the turnover of salvage biosynthesis of NAD+, which activates the SIRT1 reaction because SIRT1 (histone deacetylase) needs NAD+. Deacetylated histone molecules induce DNA silencing, contributing to anti-aging and longevity. When β-NMN is intraperitoneally injected, β-NMN is dephosphorylated in the blood to form Nam riboside (NR), which is then transported into the cells. NR is re-phosphorylated to form β-NMN, which is then synthesized to NAD+ in the nucleus. In contrast to Nam, which is controlled at the Nam→β-NMN reaction, the β-NMN biosynthesis pathway is not regulated[1].
Side Effects
NMN studies to date suggest mostly beneficial effects with minimal side effects. However, though generally well tolerated, nicotinamide can have ill effects on the kidneys, liver, and beta-cells in the pancreas (which makes insulin) and can lead to gastrointestinal upset and headaches. These effects seem to be dose-dependent, with higher doses causing negative effects and lower doses having more protective effects.
in vitro
β-nicotinamide mononucleotide has several beneficial pharmacological activities. Mostly mediated by its involvement in NAD+ biosynthesis, the pharmacological activities of NMN include its role in cellular biochemical functions, cardioprotection, diabetes, Alzheimer's disease, and complications associated with obesity.The intracellular NAD+ levels are significantly decreased by knockdown or knockout of Nampt (Nampt KD or Nampt KO) or treatment with Nampt inhibitor FK866, whereas NAD+ levels are dramatically increased by supplement of NAD+ precursors NAM or NMN (0.5–1 mM). NAD+ precursor NMN treatment inhibited CD8+ T cells activation and function.
in vivo
β-Nicotinamide mononucleotide (500 mg/kg; i.p.; 3 times per week for 7-10 week) prevents mtDNA damage and Dox-induced cardiac dysfunction.Nampt KO markedly inhibits tumor progression, whereas Nampt metabolite β-Nicotinamide mononucleotide (300 mg/kg body weight; i.p.; once every two days for 2 weeks) significantly promotes tumor growth in C57BL/6 mice (bearing wildtype Hepa1-6 cells). The reduction and increase in NAD+ level of respective Nampt KO and β-Nicotinamide mononucleotide-treated tumors are confirmed.β-nicotinamide mononucleotide ameliorates glucose intolerance by restoring NAD(+) levels in HFD-induced T2D mice. β-nicotinamide mononucleotide also enhances hepatic insulin sensitivity and restores gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation.
Purification Methods
Purify NMN by passage through a column of Dowex-1 (Clform) and washing with H2O until no absorbance is observed at 260 nm. The tubes containing NMN are pooled, adjusted to pH 5.5-6 and evaporated in vacuo to a small volume. This is adjusted to pH 3 with dilute HNO3 in an ice-bath and treated with 20volumes of Me2CO at 0-5o. The heavy white precipitate is collected by centrifugation at 0o. It is best stored wet and frozen or it can be dried to give a gummy residue. It has max 266nm ( 4,600) and min 249nm ( 3600) at pH 7.0 (i.e. no absorption at 340nm). It can be estimated by reaction with CNor hydrosulfite which form the 4-adducts (equivalent to NADH) which have UV max 340nm ( 6,200). Thus after reaction, an OD340 of one is obtained from a 0.1612mM solution in a 1cm path cuvette. [Plaut & Plaut Biochemical Preparations 5 56 1957, Maplan & Stolzenbach Methods Enzymol 3 899 1957, Kaplan et al. J Am Chem Soc 77 815 1955, Beilstein 22/2 V 168.]
References
[1] Kawamura T., et al. "β-Nicotinamide Mononucleotide, an Anti-Aging Candidate Compound, Is Retained in the Body for Longer than Nicotinamide in Rats." Journal of nutritional science and vitaminology 62(2016): 272-276.
Properties of β-Nicotinamide Mononucleotide
| Melting point: | 166 °C(dec.) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | White to Yellow |
| Merck | 13,6697 |
| BRN | 3570187 |
| Stability: | Very Hygroscopic |
| InChI | InChI=1/C11H15N2O8P/c12-10(16)6-2-1-3-13(4-6)11-9(15)8(14)7(21-11)5-20-22(17,18)19/h1-4,7-9,11,14-15H,5H2,(H3-,12,16,17,18,19)/t7-,8-,9-,11-/s3 |
Safety information for β-Nicotinamide Mononucleotide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for β-Nicotinamide Mononucleotide
| InChIKey | DAYLJWODMCOQEW-TURQNECASA-N |
| SMILES | O[C@@H]1[C@@H]([C@@H](COP(O)([O-])=O)O[C@H]1[N+]1=CC=CC(C(=O)N)=C1)O |&1:1,2,3,11,r| |
β-Nicotinamide Mononucleotide manufacturer
GLR Innovations
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 beta-Nicotinamide mononucleotide High quality high purity, size packaging, large supplyView Details
beta-Nicotinamide mononucleotide High quality high purity, size packaging, large supplyView Details
1094-61-7 -
 1094-61-7 NMN(BETA-NICOTINAMIDE MONONUCLEOTIDE) 98%View Details
1094-61-7 NMN(BETA-NICOTINAMIDE MONONUCLEOTIDE) 98%View Details
1094-61-7 -
 1094-61-7 98%View Details
1094-61-7 98%View Details
1094-61-7 -
 ß-Nicotinamide Mononucleotide (ß-NMN) for cell culture CAS 1094-61-7View Details
ß-Nicotinamide Mononucleotide (ß-NMN) for cell culture CAS 1094-61-7View Details
1094-61-7 -
 β-Nicotinamide Mononucleotide CAS 1094-61-7View Details
β-Nicotinamide Mononucleotide CAS 1094-61-7View Details
1094-61-7 -
 ß-Nicotinamide Mononucleotide (ß-NMN) extrapure CAS 1094-61-7View Details
ß-Nicotinamide Mononucleotide (ß-NMN) extrapure CAS 1094-61-7View Details
1094-61-7 -
 Beta-nicotinamide mononucleotide 95% CAS 1094-61-7View Details
Beta-nicotinamide mononucleotide 95% CAS 1094-61-7View Details
1094-61-7 -
 β-Nicotinamide mononucleotide CAS 1094-61-7View Details
β-Nicotinamide mononucleotide CAS 1094-61-7View Details
1094-61-7
