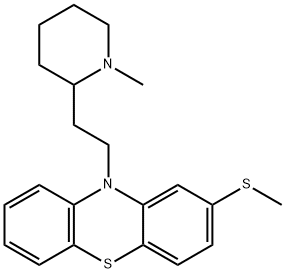
Mesoridazine Besylate
Synonym(s):10-[2-(1-Methyl-2-piperidinyl)ethyl]-2-(methylsulfinyl)-10H-phenothiazine;Mesoridazine benzenesulfonate
- CAS NO.:32672-69-8
- Empirical Formula: C27H32N2O4S3
- Molecular Weight: 544.75
- MDL number: MFCD00800983
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24

What is Mesoridazine Besylate?
Originator
Serentil,Sandoz,US,1970
The Uses of Mesoridazine Besylate
Mesoridazine (M225755) Besylate is an antipsychotic.
The Uses of Mesoridazine Besylate
antpsychotic
What are the applications of Application
Mesoridazine besylate is a D2DR and D4DR inhibitor
Definition
ChEBI: The benzenesulfonate salt of mesoridazine prepared using equimolar amounts of mesoridazine and benzenesulfonic acid.
Manufacturing Process
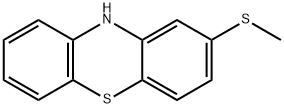
10.0 g of 3-methylmercapto phenothiazine and 17.5 cc of acetic acid
anhydride are refluxed for 8 hours from an oil bath maintained at a
temperature of 180°C. After concentration of the solution the residue is
crystallized from ethanol. The pure 3-methylmercapto-10-acetyl phenothiazine
melts at 89° to 91°C. For the purpose of oxidation 5.0 g of 3-
methylmercapto-10-acetyl phenothiazine are dissolved in 50 cc of ethanol,
refluxed from an oil bath maintained at 120°C and 1.6 cc of a 40% hydrogen
peroxide solution are then added dropwise in the course of 30 minutes.
Heating is continued for another 5 hours and the reaction mixture is
concentrated after 50 cc of water have been added. The residue is taken up in
40 cc of benzene and the benzene layer washed with 10 cc of water. After
having been concentrated, the residue, crude 3-methylsulfinyl-10-acetyl
phenothiazine, is dissolved in 55 cc of a 90% methanol solution for splitting
off the acetyl group and, after 2.9 g of potassium carbonate have been added,
it is boiled for 2 hours under reflux on an oil bath kept at a temperature of
120°C. After concentration, the residue is taken up in 50 cc of chloroform, the
chloroform layer is washed with a total of 25 cc of water, dried over potassium
carbonate, filtered and concentrated. After twice crystallizing the residue, each
time from 50 cc of ethanol, analytically pure 3-methylsulfinyl phenothiazine
(MP 193° to 195°C) is obtained.
A mixture of 10.0 g of 3-methylsulfinyl phenothiazine (MP 193° to 195°C), 6.1
g of finely powdered sodium hydroxide and 125 cc of toluene is boiled for 1
hour under reflux with a water separator on an oil bath kept at a temperature
of 150°C, while the mixture is stirred. Without interrupting the boil a solution
of 7.0 g of 2-(N-methyl-piperidyl-2')-1-chloroethane (BP 84°C/10 mm Hg) in
10 cc of toluene is added dropwise in the course of 1 hour, after which boiling
is continued for another 3 hours. When the reaction mixture has cooled it is
first washed with 25 cc of water three times and then extracted with 75 cc of
a 15% aqueous tartaric acid solution. The tartaric acid extract is shaken out
with 25 cc of benzene, 20 cc of concentrated caustic soda are added until the
phenolphthalein reaction is alkaline, and the separated oily base is taken up in
a total of 150 cc of benzene.
After having been washed with 50 cc of water the benzene layer is dried over
potassium carbonate, filtered, allowed to stand over 10 g of alumina for about
1? hours for partial decolorization, filtered again and concentrated under
reduced pressure. The oily base which remains as a residue is directly
converted into the tartrate. A solution cooled to 0°C, of 6.50 g of the free
base in 100 cc of acetic acid ethyl ester is thoroughly shaken and poured into
an ice cold solution of 2.66 g of tartaric acid in 410 cc of acetic acid ethyl
ester. The precipitated, analytically pure, tartrate of 3-methylsulfinyl-10-[2'-Nmethyl-piperidyl-2')-ethyl-l']-phenothiazine melts at 115° to 120°C (foam
formation) and sinters above 80°C. The base is reacted with benzene sulfonic
acid in a suitable solvent to give the besylate.
brand name
Serentil (Novartis).
Therapeutic Function
Tranquilizer
General Description
Mesoridazine besylate,10-[2-(methyl-2-piperidyl)ethyl]-2-(methylsulfinyl)phenothiazinemonobenzenesulfonate (Serentil), shares manyproperties with thioridazine. However, no pigmentaryretinopathy has been reported.
Properties of Mesoridazine Besylate
| Melting point: | 174-175 °C |
| storage temp. | 2-8°C |
| solubility | DMSO: 10 mg/mL |
| form | solid |
| color | white |
| Stability: | Hygroscopic |
Safety information for Mesoridazine Besylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Mesoridazine Besylate
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Mesoridazine besylate CAS 32672-69-8View Details
Mesoridazine besylate CAS 32672-69-8View Details
32672-69-8 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1