Meclozine
- CAS NO.:569-65-3
- Empirical Formula: C25H27ClN2
- Molecular Weight: 390.95
- MDL number: MFCD00242697
- EINECS: 209-323-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
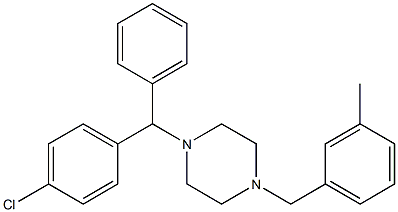
What is Meclozine?
Absorption
Most histamine H1 antagonists are reported to be readily absorbed following oral administration. Upon oral administration, the time to reach peak plasma concentrations (Cmax) of meclizine is about 3 hours post-dose, with the value ranging from 1.5 to 6 hours.
Toxicity
The oral and intraperitoneal LD50 in mouse are 1600 mg/kg and 625 mg/kg, respectively. The lowest published toxic dose (TDLo) in rats via the oral route is 800 mg/kg.
Symptoms of overdose mainly involve CNS depression with drowsiness, coma, and convulsions. Hypotension may also occur, particularly in the elderly. In children, anticholinergic effects and CNS stimulation, characterized by hallucinations, seizures, trouble sleeping, are more likely to occur. In case of overdose, symptomatic and supportive treatment is recommended. In case of recent ingestion, induction of emesis or gastric lavage should be initiated to limit further drug absorption. Although there is no known antidote to meclizine, physostigmine may be useful to counteract the CNS anticholinergic effects of meclizine.
Originator
Antivert,Roerig,US,1957
The Uses of Meclozine
Anti-emetic.
The Uses of Meclozine
Meclizine actively affects the vomiting center and is used for vomiting and diarrhea. Synonyms of this drug are antivert, bonine, lamin, roclizin, and vertol.
Indications
Indicated for the symptomatic treatment of nausea, vomiting, and dizziness associated with motion sickness, and management of vertigo due to various causes, including radiation sickness, Meniere’s syndrome, labyrinthitis and other vestibular disturbances.
Background
Meclizine is a histamine H1 antagonist with antiemetic and antivertigo properties. It is used in the symptomatic treatment of motion sickness and control of vertigo associated with vestibular system diseases. It also exhibits anticholinergic, central nervous system depressant, and local anesthetic effects. Commonly marketed under the brand name Antivert in the U.S., meclizine is available as oral tablets.
Definition
ChEBI: Meclizine is a diarylmethane.
Manufacturing Process
32.3 g of 1-p-chlorobenzhydryl-4-benzyl-piperazine, dissolved in 300 cm3 of alcohol are heated in an autoclave vessel, in the presence of Raney nickel, under a pressure of 100 kg H2, at about 150°C for 6 hours. The catalyst is filtered, the solvent is evaporated and the residue is fractionated under a high vacuum. p-Chlorobenzylhydryl-piperazine (BP 180° to 185°C/1 mm Hg) is isolated with a yield of 75%. Then finely ground NaNH2 is added. The mixture is heated under reflux for 1 hour, the mass is cooled and a molar equivalent of m-methyl benzyl chloride is added.
The solvent is evaporated and the residue is dissolved in chloroform. This
solution is washed with a saturated solution of K2CO3 and dried on K2CO3. The
solvent is evaporated and the residue is distilled under high vacuum. The
product of the condensation distills near 230°C at 2 mm Hg pressure and the
corresponding dihydrochloride melts at 217° to 224°C.
brand name
Antivert (Pfizer);Ancoloxine;Bonamina;Bonamine;Calmonal;Chiclida;Cobinamide;Diadril;Dradril;Duremesan;Itinerol;Mecazine;Navicalm;Neo-istafenc;Neo-istafene;Peremesin;Postafene;Premesin;Rovert-m;Ru-vert-m;Sea-leg;Supermesin;Superminal;Suprimal;Taizerl;V-cline;Veritab;Vertizine;Vomaxine;Vomisseis.
Therapeutic Function
Antinauseant
World Health Organization (WHO)
Meclozine, an antihistamine with antiemetic activity, was introduced in 1953 for the treatment of nausea. The action taken in Indonesia in 1963 resulted from concern regarding its possible teratogenic potential. Subsequent epidemiological studies have been widely accepted, however, as dispelling this suspicion. Meclozine remains widely available in both prescription only and over-the-counter preparations and in some countries the licensed indications include management of nausea of pregnancy.
Pharmacokinetics
Meclizine works on the higher centres of the brain to reduce nausea, vomiting, or vertigo. It is effective against nausea and vomiting arising from many causes, including motion sickness and disorders affecting the vestibular system. The onset of action of meclizine is about 1 hour, with effects lasting between 8 to 24 hours. Meclizine is reported to cause drowsiness due to its anticholinergic actions.
Synthesis
Meclizine, 1-[(4-chlorphenyl)methyl]-4-[(3-methylphenyl)phenyl]piperazine (16.1.16), is synthesized by reductive amination of a mixture of 3-methylbenzaldehyde with 1-(4-chlorbenzhydryl)piperazine using hydrogen over Raney nickel.
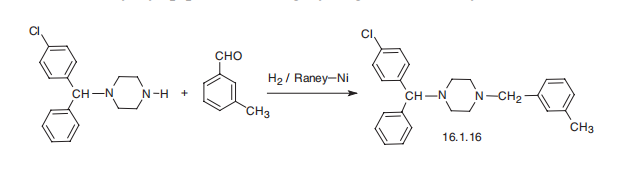
Metabolism
There is limited human data on meclizine metabolism. According to the findings of in vitro studies, meclizine may undergo aromatic hydroxylation or benzylic oxidation mediated by the hepatic CYP2D6 enzyme.
Properties of Meclozine
| Boiling point: | bp2 230° |
| Density | 1.0318 (rough estimate) |
| refractive index | 1.5940 (estimate) |
| storage temp. | 2-8°C |
| pka | pKa 2.05(H2O,t =25,I=0.0051(NaCl)) (Uncertain) |
| CAS DataBase Reference | 569-65-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Meclizine(569-65-3) |
| EPA Substance Registry System | Meclizine (569-65-3) |
Safety information for Meclozine
Computed Descriptors for Meclozine
Meclozine manufacturer
Sri Krishna Pharmaceuticals Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 569-65-3 Meclizine 98%View Details
569-65-3 Meclizine 98%View Details
569-65-3 -
 569-65-3 98%View Details
569-65-3 98%View Details
569-65-3 -
 Meclizine 569-65-3 98%View Details
Meclizine 569-65-3 98%View Details
569-65-3 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0