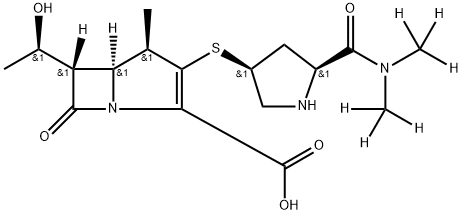
Meropenem Trihydrate
Synonym(s):(1R,5S,6S)-2-[(3S,5S)-5-(dimethylaminocarbonyl)pyrrolidin-3-ylthio]-6-[(R)-1-hydroxyethyl]-1-methylcarbapen-2-em-3-carboxylic acid trihydrate
- CAS NO.:119478-56-7
- Empirical Formula: C17H27N3O6S
- Molecular Weight: 401.48
- MDL number: MFCD00864966
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-13 11:39:26
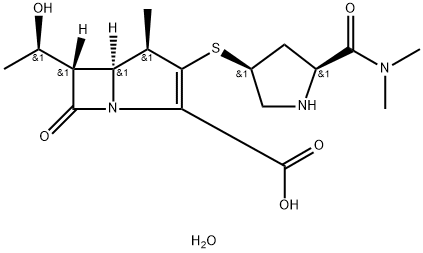
What is Meropenem Trihydrate?
Chemical properties
White or slight-yellow crystalline powder LOD ≤0.5% Heavy Metals ≤20 ppm
The Uses of Meropenem Trihydrate
Meropenem trihydrate is a carbapenem antibiotic with wide spectrum of antibacterial. It is an ultra-broad spectrum beta-lactam antibiotic and has antibacterial activity against Gram-negative and Gram-positive bacteria as well as anaerobic microorganisms such as Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter species, Serratia marcescens, Streptococcus pyogenes, Streptococcus agalactiae and Citrobacter sp. Meropenem trihydrate is indicated for the treatment of serious bacterial infection including complicated skin infections, complicated intra-abdominal infections, urinary tract infections, severe pneumonia, broncho-pulmonary infections and bacterial meningitis.
Definition
ChEBI: Meropenem trihydrate is a hydrate. It has a role as an antibacterial drug. It contains a meropenem.
brand name
Merrem I.V. (AstraZeneca).
Antimicrobial activity
The unique side chain at C-2 is associated with increased activity against Gram-negative bacteria, including H. influenzae. It is slightly less active than imipenem against Gram-positive organisms. It is active against anaerobes and more active against some strains that are less susceptible to imipenem. Its excellent activity against Gram-negative organisms is due to high affinity for multiple penicillin-binding proteins. Activity is little affected by inoculum size or the presence of serum. It is bactericidal at concentrations close to the MIC.
Stability to β-lactamases is similar to that of other carbapenems: it is highly resistant to most serine β-lactamases, including extended-spectrum enzymes, but can be hydrolyzed by metallo-β-lactamases and by serine carbapenemases.
General Description
Meropenem trihydrate (MRP) belongs to the carbapenem group of compounds. It is susceptible to degradation at high temperature and humidity. It is soluble in methanol-water or acetone-water combinations. It is used as an antibacterial agent for treating meningitis and pneumonia.
Biological Activity
meropenem trihydrate (sm-7338) is an active ingredient carbapenem antibiotic [1].meropenem trihydrate has shown the potent effect against gram-negative organisms with the mic90 values of 0.03μm, 0.06μm, 0.06μm, 0.12μm,0.25μm, 0.12μm, 0.06μm, 0.12μm and 0.25μm for escherichia coli (30), klebsiella pneumonia(29), klebsiella oxytoca (20), enterobacter aerogenes (14), enterobacter cloacae (29), citrobacter freundii (20), citrobacter diversus (12), proteus mirabilis (15) and morganella morganii (15), respectively. in addition, meropenem trihydrate has also been revealed to restrain gram-positive and anaerobic organisms with the mic90 values of 0.008μm, 4μm and 0.015μm for streptococcus pyogenes (20), viridans group streptococci (27), streptococcus pneumonia (15), respectively. furthermore, meropenem trihydrate has shown the different effect of ph on mic, for example, the mean mic values of 0.06μm and 0.03μm in ph5.5 and ph7.5, respectively [1].
Biochem/physiol Actions
Meropenem trihydrate is an ultra-broad spectrum beta-lactam antibiotic active against both Gram-positive and Gram-negative bacteria.
Pharmacokinetics
Cmax 500 mg intravenous (30-min
infusion): 23 mg/L end infusion
1 g intravenous (30-min infusion): 49 mg/L end infusion
Plasma half-life: 1 h
Volume of distribution: c. 0.3 L/kg
Plasma protein binding: 2%
Absorption and distribution
Meropenem is not absorbed after oral administration. It
penetrates well into most body fluids and tissues, including
CSF, achieving concentrations matching or exceeding those
required to inhibit most susceptible bacteria. In pediatric
patients (1 month to 15 years) with inflamed meninges it
achieves CSF levels of 0.9–6.5 mg/L after a single intravenous
infusion (40 mg/kg) over 30 min. After a single intravenous
dose, the highest mean concentrations of meropenem were
found in tissues and fluids at 1 h (0.5–1.5 h) after the start
of infusion.
Metabolism and excretion
The mean recovery of unchanged meropenem is approximately
70%. The remainder consists of the microbiologically
inactive open-ring form. Renal excretion is greater than
70% of unchanged drug over 12 h. Co-administration with
probenecid prolongs the half-life 38%, but peak concentrations
are not greatly affected. In patients with renal impairment
the dose should be adjusted. Parent drug and metabolite
are removed by hemodialysis.
Indications
Intra-abdominal infections
Bacterial meningitis (pediatric patients >3 months)
Complicated skin and skin structure infections
Clinical Use
Meropenem is a second-generation carbapenem that, todate, has undergone the most extensive clinical evaluation.It has recently been approved as Merrem for the treatmentof infections caused by multiply-resistant bacteria and forempirical therapy for serious infections, such as bacterialmeningitis, septicemia, pneumonia, and peritonitis.Meropenem exhibits greater potency against Gram-negativeand anaerobic bacteria than does imipenem, but it is slightlyless active against most Gram-positive species. It is not effectiveagainst MRSA. Meropenem is not hydrolyzed byDHP-I and is resistant to most β-lactamases, including a fewcarbapenemases that hydrolyze carbapenem.
Side Effects
Seizures and other CNS adverse experiences have been
reported in 0.7% of all adult patients, most commonly those
with pre-existing CNS disorders. Pseudomembranous colitis
has been reported. Other reactions include diarrhea (4.8%),
nausea and vomiting (3.6%), inflammation at the site of
injection (2.4%) and headache (2.3%). Moniliasis occurs in
1.9–3.1% of pediatric patients.
Patients with a history of hypersensitivity reactions to other
β-lactam agents should be treated cautiously.
Drug interactions
Potentially hazardous interactions with other drugs
Antiepileptics: concentration of valproate reduced -
avoid.
Probenecid: avoid concomitant use
Metabolism
Meropenem is more stable to renal dehydropeptidase I than imipenem but undergoes some renal metabolism, and is mainly excreted in the urine by tubular secretion and glomerular filtration. About 70% of a dose is recovered unchanged in the urine over a 12-hour period. Meropenem is reported to have one metabolite (ICI 213689), which is inactive and is excreted in the urine.
References
[1] neu hc1, novelli a, chin nx.in vitro activity and beta-lactamase stability of a new carbapenem, sm-7338.antimicrob agents chemother. 1989 jul; 33(7):1009-18.
Properties of Meropenem Trihydrate
| Melting point: | >192°C (dec.) |
| Boiling point: | 627℃ |
| alpha | -17~-21゜(d/20℃)(c=0.5,H2O)(calculated on the dehydrous basis) |
| RTECS | CL5446509 |
| Flash point: | >110°(230°F) |
| storage temp. | -20°C |
| solubility | Soluble in aqueous solution at approximately 5mg/ml |
| form | powder |
| color | white to off-white |
| Merck | 14,5900 |
| CAS DataBase Reference | 119478-56-7(CAS DataBase Reference) |
Safety information for Meropenem Trihydrate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for Meropenem Trihydrate
| InChIKey | DMJNNHOOLUXYBV-PQTSNVLCSA-N |
| SMILES | C(C1=C([C@H](C)[C@]2([H])[C@@]([H])([C@H](O)C)C(=O)N12)S[C@@H]1CN[C@H](C(=O)N(C)C)C1)(=O)O.O |&1:3,5,7,9,16,19,r| |
Abamectin manufacturer
Rivashaa Agrotech Biopharma Pvt. Ltd.
Ralington Pharma
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
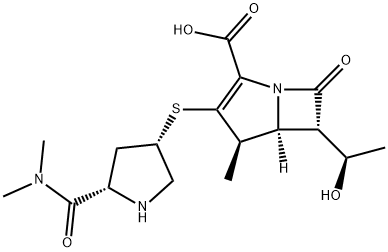

You may like
-
 Meropenem 98%View Details
Meropenem 98%View Details
119478-56-7 -
 119478-56-7 Meropenem trihydrate 99%View Details
119478-56-7 Meropenem trihydrate 99%View Details
119478-56-7 -
 Meropenem trihydrate 96% CAS 119478-56-7View Details
Meropenem trihydrate 96% CAS 119478-56-7View Details
119478-56-7 -
 119478-56-7 95-99%View Details
119478-56-7 95-99%View Details
119478-56-7 -
 Meropenem Trihydrate CAS 119478-56-7View Details
Meropenem Trihydrate CAS 119478-56-7View Details
119478-56-7 -
 Meropenem CAS 119478-56-7View Details
Meropenem CAS 119478-56-7View Details
119478-56-7 -
 Meropenem CAS 119478-56-7View Details
Meropenem CAS 119478-56-7View Details
119478-56-7 -
 Meropenem trihydrate CAS 119478-56-7View Details
Meropenem trihydrate CAS 119478-56-7View Details
119478-56-7