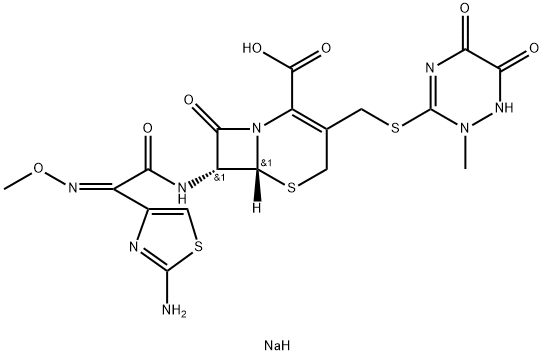
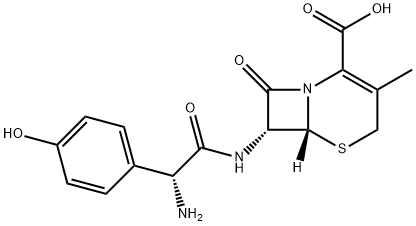
Ceftriaxone sodium
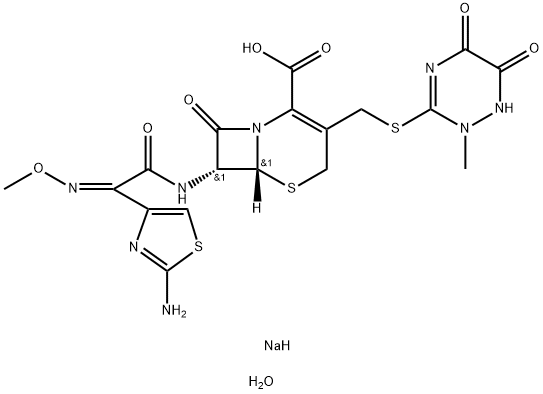
Synonym(s):Ceftriaxone disodium salt hemi(heptahydrate)
- CAS NO.:74578-69-1
- Empirical Formula: C18H19N8NaO7S3
- Molecular Weight: 578.57
- MDL number: MFCD00865013
- EINECS: 277-930-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-04 20:40:58
What is Ceftriaxone sodium?
Description
Ceftriaxone is a cephalosporin (SEF a low spor in) antibiotic that is used to treat conditions such as lower respiratory tract infections, skin and skin structure infections, urinary tract infections, pelvic inflammatory disease, bacterial septicemia, bone and joint infections, and meningitis.
Originator
Rocephin,Roche,Switz.,1982
The Uses of Ceftriaxone sodium
Ceftriaxone sodium is antibacteria,an impurity of Ceftriaxone (C244995). It is an antibacterial, a third-generation cephalosporin.
brand name
Rocephin (Roche).
Therapeutic Function
Antibacterial
General Description
Ceftriaxone was synthesized by HoffmannLa Roche in 1981. The triazinyl moiety was introduced at the 3 position of the cephem nucleus. The same side chain as possessed by cefotaxime and the other so-called third-generation cephalosporins was retained at the 7 position. The antibacterial activity of ceftiaxone is almost the same as that of cefotaxime in vitro, but its in vivo activity is 10 to 100 times higher. Its most characteristic property is its seven to eight hour half-life in serum, the longest among the known cephem antibiotics.
Hazard
Moderately toxic. Low toxicity by inges- tion. Human systemic effects.
Mechanism of action
Ceftriaxone is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Ceftriaxone has activity in the presence of some beta-lac- tamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria. Ceftriaxone works by inhibiting the mucopeptide synthesis in the bacterial cell wall. The β-lactam core of ceftriaxone binds to carboxypeptidases, endopeptidases, and transpeptidases in the bacterial cytoplasmic membrane. These enzymes are involved in cell wall synthesis and cell division. By binding to these enzymes, ceftriaxone causes formation of defective cell walls and promotes cell death[1].
Clinical Use
Ceftriaxone sodium is a β-lactamase–resistantcephalosporin with an extremely long serum half-life.Once-daily dosing suffices for most indications. Two factorscontribute to the prolonged duration of action ofceftriaxone: high protein binding in the plasma and slowurinary excretion. Ceftriaxone is excreted in both the bileand the urine. Its urinary excretion is not affected byprobenecid. Despite its comparatively low volume ofdistribution, it reaches the cerebrospinal fluid in concentrationsthat are effective in meningitis. Nonlinear pharmacokineticsare observed.
Ceftriaxone contains a highly acidic heterocyclic systemon the 3-thiomethyl group. This unusual dioxotriazine ringsystem is believed to confer the unique pharmacokineticproperties of this agent. Ceftriaxone has been associatedwith sonographically detected “sludge,” or pseudolithiasis,in the gallbladder and common bile duct. Symptoms ofcholecystitis may occur in susceptible patients, especiallythose on prolonged or high-dose ceftriaxone therapy. Theculprit has been identified as the calcium chelate.
Ceftriaxone exhibits excellent broad-spectrum antibacterialactivity against both Gram-positive and Gram-negativeorganisms. It is highly resistant to most chromosomally andplasmid-mediated β-lactamases. The activity of ceftriaxoneagainst Enterobacter, Citrobacter, Serratia, indole-positiveProteus, and Pseudomonas spp. is particularly impressive. Itis also effective in the treatment of ampicillin-resistant gonorrheaand H. influenzae infections but generally less activethan cefotaxime against Gram-positive bacteria and B.fragilis.
Side Effects
Common side effects of Ceftriaxone sodium include:
symptoms of a blood cell disorder;
diarrhea;
vaginal itching or discharge;
warmth, tight feeling, or a hard lump where the injection was given;
rash;
abnormal liver function tests.
Side Effects
Ceftriaxone sodium is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Ceftriaxone has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
Synthesis
A solution of sodium-2-ethyl hexanoate (390 g) in acetone (2.0 Ltr) is added to the wet Ceftriaxone acid obtained in Example 2 is suspended in a mixture of acetone and water. The reaction mixture is charcoalized and filtered. To the clear filtrate is added acetone whereupon the product precipitated. The precipitated solid is filtered, washed with acetone and dried to get 515 g of Ceftriaxone sodium hemiheptahydrate. HPLC purity = Above 99.5%.
Veterinary Drugs and Treatments
Ceftriaxone is used to treat serious infections, particularly against susceptible Enterobacteriaceae that are not susceptible to other less expensive agents or when aminoglycosides are not indicated (due to their potential toxicity). Its long half life, good CNS penetration, and activity against Borrelia burgdorferi also has made it a potential choice for treating Lyme’s disease.
storage
+4°C
References
[1] Rawls S, et al. Antibiotics, β-Lactam. Encyclopedia of the Neurological Sciences, 2014; 207-209.
Properties of Ceftriaxone sodium
| storage temp. | 4°C, protect from light |
| solubility | Soluble in DMSO (50 mM) |
| form | Solid |
| color | White to off-white |
| Water Solubility | Water : ≥ 40 mg/mL (66.60 mM) |
| CAS DataBase Reference | 74578-69-1(CAS DataBase Reference) |
Safety information for Ceftriaxone sodium
Computed Descriptors for Ceftriaxone sodium
| InChIKey | CDCJSJLFRBHEHN-ZZKDXJNYNA-N |
| SMILES | C(C1=C(CS[C@]2([H])[C@H](NC(=O)/C(/C3N=C(N)SC=3)=N\OC)C(=O)N12)CSC1=NC(=O)C(=O)NN1C)(=O)O.[NaH] |&1:5,7,r| |
Ceftriaxone sodium manufacturer
AKASH PHARMA EXPORTS
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 74578-69-1 Ceftriaxone disodium 98%View Details
74578-69-1 Ceftriaxone disodium 98%View Details
74578-69-1 -
 74578-69-1 98%View Details
74578-69-1 98%View Details
74578-69-1 -
 Ceftriaxone Sodium 99%View Details
Ceftriaxone Sodium 99%View Details -
 Ceftriaxone sodium CAS 74578-69-1View Details
Ceftriaxone sodium CAS 74578-69-1View Details
74578-69-1 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
