COBALT(III) OXIDE BLACK
Synonym(s):Cobalt tetraoxide;Cobaltic-cobaltous oxide;Tricobalt tetraoxide
- CAS NO.:1308-04-9
- Empirical Formula: Co2O3
- Molecular Weight: 165.86
- MDL number: MFCD00036266
- EINECS: 215-156-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
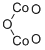
What is COBALT(III) OXIDE BLACK?
Description
Neither the oxide Co2O3 nor the hydroxide Co(OH)3 is definitely established. The oxidation of cobalt(II) hydroxide in aqueous suspension with, for example, peroxides, or the destruction of a cobalt(III) complex with alkali gives a brown or black powder Co2O3.aq which upon drying at 150° yields the monohydrate Co2O3.H2O; this is probably best formulated as CoO(OH). When heated further in attempts to dehydrate it, the "monohydrate" begins to evolve oxygen (as well as water) at 300° with the formation of black Co3O4. When heated to 100° in air cobalt(II) hydroxide is converted to dark brown CoO(OH).
Chemical properties
Steel-gray or black powder.Soluble in concentrated acids; insoluble in water.
Physical properties
Grayish black powder; density 5.18 g/cm3; decomposes at 895°C; insoluble in water; soluble in concentrated mineral acids.
The Uses of COBALT(III) OXIDE BLACK
Pigment, coloring enamels, glazing pottery.
The Uses of COBALT(III) OXIDE BLACK
Cobaltic oxide (Co2O3) is also known as cobalt oxide or cobalt black. It is dark gray to black and is used in pigments, ceramic glazes, and semiconductors.
Definition
Sometimes incorrectly called cobalt peroxide.
Preparation
Cobalt(III) oxide is prepared by heating cobalt compounds at low temperatures in air.
Safety Profile
: Moderately toxic by intraperitoneal and subcutaneous routes. Questionable carcinogen. Violent reaction with hydrogen peroxide. The oxide increases the sensitivity of nitroalkanes (e.g., nitromethane, nitroethane, and 1 nitropropane) to heat or detonation. See also COBALT COMPOUNDS.
Properties of COBALT(III) OXIDE BLACK
| Melting point: | 895 °C (dec.)(lit.) |
| Density | 6.11 g/mL at 25 °C(lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | insoluble in H2O; soluble in concentrated acid solutions |
| form | powder |
| color | gray-black |
| Water Solubility | insoluble H2O; soluble conc acids [HAW93] |
| EPA Substance Registry System | Cobalt oxide (Co2O3) (1308-04-9) |
Safety information for COBALT(III) OXIDE BLACK
Computed Descriptors for COBALT(III) OXIDE BLACK
Related products of tetrahydrofuran

You may like
-
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 99-30-9 99%View Details
99-30-9 99%View Details
99-30-9 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 98-56-6 99%View Details
98-56-6 99%View Details
98-56-6 -
 694-48-4 99%View Details
694-48-4 99%View Details
694-48-4 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3 -
 Methyl chloroacetate, 98% 96-34-4 99%View Details
Methyl chloroacetate, 98% 96-34-4 99%View Details
96-34-4