Magnesium iodide
Synonym(s):Magnesium diiodide
- CAS NO.:14332-62-8
- Empirical Formula: MgI2
- Molecular Weight: 278.11
- MDL number: MFCD00049485
- EINECS: 233-825-1
- SAFETY DATA SHEET (SDS)
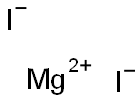
What is Magnesium iodide?
Physical properties
MgI2·8H2O is a crystalline powder, white and deliquescent,
discoloring in air; soluble in water, alcohol, and
ether. This salt has been used in medicine. The anhydrous
compound has a molecular weight of 278.1139 g/mol,a melting point of 637°C, a density of 4.43 g/cm3 and
a water solubility of 1480 g/l at 18°C. MgI2 has the
same type of structure (CdI2) as the chloride and bromide
except that the cell parameters differ.
Magnesium iodide is stable at high heat under
a hydrogen atmosphere, but decomposes in air at normal temperatures, turning brown due to the release
of elemental iodine. When heated in air, it decomposes
completely to magnesium oxide, releasing iodine
vapor.
Magnesium iodide is known to form three
hydrates, MgI2·6H2O, MgI2·8H2O and MgI2·10H2O of which the decahydrate is claimed to be the most stable.
The Uses of Magnesium iodide
Magnesium iodide has few commercial uses but can be used to prepare many compounds for organic synthesis. Usage of magnesium iodide in the “Baylis– Hillman” reaction (an organic reaction of an aldehyde and an α,β-unsaturated electron-withdrawing group catalyzed by MgI2) tends to give vinyl-specific compounds. Another example of the usage of MgI2 as a catalyst involves the reaction of 2,3,4,6-tetra-O-benzyl-D-gluconolactone or 2,3,4,6-tetra-O-benzyl-D-mannono-lactone with MgI2 in EtOH which afforded the respective C-2 epimer.
Preparation
Magnesium iodide is the name for the chemical compounds with the formulas MgI2 and its various hydrates Mgl2(H20)X. lt has few commercial uses, but can be used to prepare compounds for organic synthesis. Magnesium iodide can be produced from magnesium oxide, magnesium hydroxide, and magnesium carbonate by reaction with hydroiodic acid:
MgO+ 2HI →MgI2+ H2O
Mg(OH)2+ 2HI → Mgl2+2H2O
MgCO3+2HI →MgI2+ CO2+ H2O
Safety information for Magnesium iodide
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 3-Nitrobenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

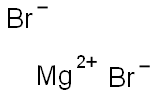
You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1