Lithium bromide
Synonym(s):Lithium monobromide
- CAS NO.:7550-35-8
- Empirical Formula: BrLi
- Molecular Weight: 86.85
- MDL number: MFCD00011077
- EINECS: 231-439-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-14 09:41:11

What is Lithium bromide?
Description
Lithium bromide is an extremely hygroscopic chemical commonly used in absorption chillers, where water is the refrigerant. Because of its high affinity to water, LiBr can easily absorb excess vapor, making the system an environmentally friendly non-CFC alternative.
Chemical properties
White cubic crystals; hygroscopic; refractive index 1.784; density 3.464 g/cm3; melts at 550°C; vaporizes at 1,265°C; highly soluble in water (145g/100g at 4°C), much greater solubility in hot water (254g/100g at 90°C); soluble in methanol, ethanol and ether; slightly soluble in pyridine; vapor pressure of aqueous solutions at 52 and 68% concentrations at 25°C are 5 and 0.7 torr, respectively.
Physical properties
White cubic crystals; hygroscopic; refractive index 1.784; density 3.464 g/cm3; melts at 550°C; vaporizes at 1,265°C; highly soluble in water (145g/100g at 4°C), much greater solubility in hot water (254g/100g at 90°C); soluble in methanol, ethanol and ether; slightly soluble in pyridine; vapor pressure of aqueous solutions at 52 and 68% concentrations at 25°C are 5 and 0.7 torr, respectively.
The Uses of Lithium bromide
As humectant, in air-conditioning systems. An aqueous solution of lithium bromide containing about 50 % lithium bromide is used as an absorbent in large-scale absorption air conditioning equipment. Lithium bromide is also used in limited amounts by the pharmaceutical industry for dehydrobromination of organic compounds to form olefins.
The Uses of Lithium bromide
Lithium Bromide finds use as a desiccant in industrial air conditioning systems and industrial drying agents.
The Uses of Lithium bromide
It is used for reconstitution of brines, as swelling agent for proteins and as electrolyte component in lithium battery. Used as brazing and welding flux. Lithium bromide is used as catalyst for the condensation of carbonyl compounds with active methylene compounds in the absence of solvent, operating medium for air-conditioning and industrial drying system. Also used in manufacturing pharmaceuticals and alkylation process. It also plays an important role in the synthesis of dihydropyrimidinones.
What are the applications of Application
Lithium bromide is an inexpensive catalyst that opens epoxide rings by amines at room temperature
Definition
ChEBI: A lithium salt in which the counterion is bromide. The anhydrous salt forms cubic crystals similar to common salt.
Preparation
Lithium bromide may be prepared by the reaction of lithium hydroxide monohydrate or lithium carbonate with hydrobromic acid. The resultant solution of lithium bromide may be evaporated and cooled to crystallize a hydrate of lithium bromide, the composition of which depends on the final temperature. The lithium bromide hydrate may be filtered from the mixture and carefully dried by the application of heat in a vacuum desiccator to avoid melting the hydrate.
General Description
LiBr/Chlorotrimethylsilane reagent participates in the conversion of alcohols to bromides.
Flammability and Explosibility
Non flammable
Purification Methods
Crystallise it several times from water or EtOH, then dry it under high vacuum for 2 days at room temperature, followed by drying at 100o. Its solubility in H2O is 167% at ~20o, and 250% at ~100o. It is deliquescent and should be stored in a tightly stoppered vessel.
Properties of Lithium bromide
| Melting point: | 550 °C (lit.) |
| Boiling point: | 1265 °C |
| Density | 1.57 g/mL at 25 °C |
| Flash point: | 1265°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Methanol (Very Slightly, Heated), Water (Slightly) |
| appearance | White solid |
| form | powder |
| pka | 2.64[at 20 ℃] |
| Specific Gravity | 3.464 |
| color | White |
| Odor | odorless |
| Water Solubility | 61 g/100 mL (25 º C) |
| Sensitive | Hygroscopic |
| Merck | 14,5526 |
| Stability: | Stable. Anhydrous form is extremely hygroscopic. |
| CAS DataBase Reference | 7550-35-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Lithium bromide(7550-35-8) |
| EPA Substance Registry System | Lithium bromide (7550-35-8) |
Safety information for Lithium bromide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lithium bromide
Lithium bromide manufacturer
JSK Chemicals
Zama Chemical
Abhishek Impex
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
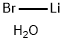

You may like
-
 Lithium Bromide 1310-06-3 98%View Details
Lithium Bromide 1310-06-3 98%View Details
1310-06-3 -
 LITHIUM BROMIDE 99%View Details
LITHIUM BROMIDE 99%View Details -
 Lithium bromide 99%View Details
Lithium bromide 99%View Details -
 Lithium bromide 99%View Details
Lithium bromide 99%View Details -
 Lithium bromide, ultra dry CAS 7550-35-8View Details
Lithium bromide, ultra dry CAS 7550-35-8View Details
7550-35-8 -
 Lithium bromide, ultra dry CAS 7550-35-8View Details
Lithium bromide, ultra dry CAS 7550-35-8View Details
7550-35-8 -
 Lithium bromide CAS 7550-35-8View Details
Lithium bromide CAS 7550-35-8View Details
7550-35-8 -
 lithium bromide CASView Details
lithium bromide CASView Details
