Ammonium hydroxide
Synonym(s):Ammonia aqueous;Ammonia Solution;Ammonia water;Ammonium hydroxide solution;Ammonium hydroxide solution, Ammonia water
- CAS NO.:1336-21-6
- Empirical Formula: H5NO
- Molecular Weight: 35.05
- MDL number: MFCD00066650
- EINECS: 215-647-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:25
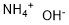
What is Ammonium hydroxide?
Description
Ammonium hydroxide is a colorless, liquid solution with a characteristic and pungent odor. It is ammonia combined with water. Ammonia (NH3) is a compound consisting of nitrogen and hydrogen. Both ammonia and ammonium hydroxide are very common compounds, found naturally in the environment (in air, water, and soil) and in all plants and animals, including humans. Ammonia is a source of nitrogen, an essential element for plants and animals. Ammonia is also produced by the human body – by our organs and tissues and by beneficial bacteria living in our intestines.
Ammonia plays an important role in protein synthesis in the human body. In brief summary, all living things need proteins, which are comprised of some 20 different amino acids. While plants and microorganisms can synthesize most amino acids from the nitrogen in the atmosphere, animals cannot. For humans, some amino acids cannot be synthesized at all and must be consumed as intact amino acids. Other amino acids, however, can be synthesized by microorganisms in the gastrointestinal tract with the help of ammonia ions. Thus, ammonia is a key player in the nitrogen cycle and in protein synthesis. Ammonia also helps maintain the body's pH balance.
Description
Ammonium hydroxide (NH4OH) solutions are basic, with a 1M NH3 solution having a pH of 11.63. Saturated NH4OH solutions contain 35% ammonia (by mass), however, concentrated solutions that are used in organic chemistry labs are typically 28-30% ammonia (by mass). The concentration of ammonia decreases slightly every time a bottle is opened due to evaporation of ammonia, especially if the bottle was removed from a refrigerator and allowed to warm to room temperature.
Chemical properties
Ammonium hydroxide exists only in the form of an aqueous solution. The compound is prepared by dissolving NH3 in H2O and usually is referred to in industrial trade as aqua ammonia. For industrial procurements, the concentration of NH3 in solution is normally specified in terms of the specific gravity (degrees Baum′e, °Be). Common concentrations are 20 °Be and 26 °Be. The former is equivalent to a sp gr of 0.933, or a concentration of about 17.8% NH3 in solution; the latter is equivalent to a sp gr of 0.897, or a concentration of about 29.4% NH3. These figures apply at a temperature of 60 °F (15.6 °C). Reagent grade NH4OH usually contains approximately 58% NH4OH (from 28 to 30% NH3 in solution).
The Uses of Ammonium hydroxide
Ammonium hydroxide is used as a cleaning agent and sanitizer in many household and industrial cleaners. Ammonium hydroxide is also used in the manufacture of products such as fertilizer, plastic, rayon and rubber. Aqueous ammonia is corrosive to aluminum alloys, copper, copper alloys, and galvanized surfaces. Aqueous ammonia is an excellent acid neutralizer.
The Uses of Ammonium hydroxide

To a solution of the SM (50.0 g, 218.0 mmol) in dry DCM (400 mL) at 0 C was added TEA (44.1 g, 437 mmol), followed by isobutyl chloroformate (596.0 g, 437.0 mmol). The reaction mixture was stirred at RT for 1 h, after which time it was concentrated in vacuo. The resulting material was cooled to 0 C and treated with NH4OH. The resulting solids were filtered, washed with H2O, and dried to provide the product as a white solid. [40.0 g, 80%]
The Uses of Ammonium hydroxide
Ammonium Hydroxide is an alkaline that is a clear, colorless solu- tion of ammonia which is used as a leavening agent, a ph control agent, and a surface finishing agent. it is used in baked goods, cheese, puddings, processed fruits, and in the production of caramels.
The Uses of Ammonium hydroxide
Ammonium hydroxide is widely utilized as a leavening agent or acidity regulator in food production. It serves as a precursor to some alkyl amines and is also used in the tobacco industry for flavor enhancement and as a processing aid. During furniture making, it combines with tannic acid and is used to darken or stain wood by making it iron salts. In chemical laboratories, it used for qualitative inorganic analysis, as a complexant and as a base. It is used to clean gold, silve, and platinum jewelry. It is an active component of Tollens' reagent (consisting of a solution of silver nitrate and ammonia) and is used to determine the presence of aldehyde or alpha-hydroxy ketone functional groups.
What are the applications of Application
Ammonium hydroxide solution is a complexant and base for traditional qualitative inorganic analysis
Definition
Ammonium hydroxide,NH40H, is a hydrate of anunonia and exists in crystalline form at -79°C. Normally, it is only found in an aqueous solution also known as aquaanunonia and anunonia water. It is prepared by dissolving NH3 inH20. Reagent grade anunonium hydroxide contains from 28 to 30% NH3 at 15.6 °C. Industrial sales specify the concentration of NH3 in solution in terms of specific gravity. Common concentrations are 20 °Be, which would bea concentration of 17.8% NH3 (specific gravity 0.933) and 26 °Be (specific gravity 0.897), or a concentration of 29.4% NH3. Ammonium hydroxide is an excellent medium for the reaction of NH3 (which becomes the NH4 radical in solution) with other compounds for the preparation of anunonium salts and other nitrogen-containing chemicals. It is an ingredientin deodorants, etching compounds, and cleaning and bleaching materials. Ammoniumhydroxide, as aqua ammonia, finds wide use as a neutralizing agent,because it is inexpensive and strongly alkaline.
What are the applications of Application
General Description
Ammonium hydroxide is a colorless aqueous solution. Concentration of ammonia ranges up to approximately 30%. Ammonia vapors (which arise from the solution) irritate the eyes.
Air & Water Reactions
Water soluble. Generates a small amount of heat when diluted with water.
Reactivity Profile
Ammonium hydroxide reacts exothermically with acids. Evolves toxic gaseous ammonia with strong bases. Reacts extremely violently with dimethyl sulfate [NFPA 491M 1991]. Reacts with aqueous silver nitrate sodium hydroxide to give a black precipitate of silver nitride. Such a precipitate can explode on stirring [MCA Case History 1554 1968]. Aqueous ammonia and Hg react to form an explosive solid, likely a fulminate. (Thodos, G. Amer. Inst. Chen. Engrs. J., 1964, 10, 274.).
Hazard
Liquid and vapor extremely irritating, especially to eyes.
Health Hazard
Ammonium hydroxide solutions are alkaline solutions, meaning they have high pH level. As a result, ammonium hydroxide is a severe eye, skin, and respiratory tract irritant, and readily burns tissue with which it comes in contact. Splashes to the eye may be serious, as contact may cause severe burns, irritation pain and possibly blindness. Direct contact with skin may cause severe burns if the chemical is not quickly rinsed away with copious amounts of water. Inhaling mists of ammonium hydroxide may result in irritation of the nose and throat with symptoms including burning, coughing, choking and pain. Inhaling concentrated mist may result in pulmonary edema and shock. Ingesting ammonium hydroxide may cause pain and burns of the esophagus and gastrointestinal tract.
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Ammonia vapor is slightly flammable (NFPA rating = 1) and ignites only with difficulty. Ammonia forms explosive mixtures with air in the range 16 to 25%. Water, carbon dioxide, or dry chemical extinguishers should be used for ammonia fires.
Agricultural Uses
Ammonium hydroxide is also known as ammonia solution, aqua ammonia, aqueous ammonia or ammonia liquor. It is the solution of ammonia in water and is commonly referred to as ammonium hydroxide. It is the simplest nitrogen solution made by forcing compressed ammonia (anhydrous ammonia) gas into water.
Safety Profile
A human poison by ingestion. An experimental poison by inhalation and ingestion. A severe eye irritant. Human systemic irritant effects by ocular and inhalation routes. Mutation data reported. Incompatible with acrolein, nitromethane, acrylic acid, chlorosulfonic acid, dimethyl sulfate, halogens, (Au + aqua regia), HCl, HF, HNO3, oleum, ppropiolactone, propylene oxide, AgNO3, Ag2O, (Ag20 + C2H5OH), AgMn04, H2SO4. Dangerous; liquid can inflict burns. Use with adequate ventilation. When heated to decomposition it emits NH3 and NO2.
Potential Exposure
It is used in detergents, stain removers, bleaches, dyes, fibers, and resins.
Storage
All work with this substance should be
conducted in a fume hood to prevent exposure by inhalation, and splash goggles and
impermeable gloves should be worn at all times to prevent eye and skin contact.
Containers should be tightly sealed to prevent escape of vapor and should be stored
in a cool area separate from halogens, acids, and oxidizers. Containers stored in
warm locations may build up dangerous internal pressures of ammonia gas.
Shipping
UN2672 Ammonia solutions, relative density between 0.880 and 0.957 at 15 C in water, with .10% but not .35% ammonia, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
Solution is strongly alkaline. Violent reaction with strong oxidizers, acids (exothermic reaction with strong mineral acids). Shock-sensitive compounds may be formed with halogens, mercury oxide; silver oxide. Fire and explosions may be caused by contact with β-propiolactone, silver nitrate; ethyl alcoho; silver permanganate; trimethylammonium amide; 1-chloro-2,4-dinitrobenzene, o-chloronitrobenzene, platinum, trioxygen difluoride; selenium difluoride dioxide; boron halides; mercury, chlorine, iodine; bromine, hypochlorites, chlorine bleach; amides, organic anhydrides; isocyanates, vinyl acetate; alkylene oxides; epichlorohydrin; aldehydes. Attacks some coatings, plastics and rubber. Attacks copper, brass, bronze, aluminum, steel, zinc, and their alloys.
Waste Disposal
Dilute with water, neutralize with HCl and discharge to sewer.
Properties of Ammonium hydroxide
| Melting point: | -77°C |
| Boiling point: | 36°C |
| Density | 0.91 g/mL at 20 °C |
| vapor density | 1.2 (vs air) |
| vapor pressure | 115 mmHg at 20 °C for 29% solution |
| storage temp. | 2-8°C |
| solubility | Water (Soluble) |
| form | Liquid, Single Sub-Boiling Quartz Distillation |
| pka | 9.3(at 25℃) |
| appearance | Colorless liquid |
| color | Colorless |
| Specific Gravity | approximate 0.96 (10%, 15℃) |
| PH | 10.09(1 mM solution);10.61(10 mM solution);11.12(100 mM solution); |
| Odor | Strong pungent ammonia odor detectable at 17 ppm |
| explosive limit | 27% |
| Water Solubility | Miscible with water. |
| λmax | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,494 |
| BRN | 3587154 |
| Stability: | Stable. Incompatible with copper, copper alloys, acids, galvanised iron, zinc, aluminium, bronze, dimethyl sulphate, mercury, alkali metals. |
| CAS DataBase Reference | 1336-21-6(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium hydroxide (1336-21-6) |
Safety information for Ammonium hydroxide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ammonium hydroxide
| InChIKey | VHUUQVKOLVNVRT-UHFFFAOYSA-N |
Ammonium hydroxide manufacturer
JSK Chemicals
Cynor Laboratories
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

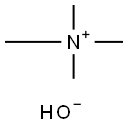
You may like
-
 Ammonia Solution 99%View Details
Ammonia Solution 99%View Details -
 Buffer solution, Colored Blue, Specpure™, NIST Traceable CAS 1336-21-6View Details
Buffer solution, Colored Blue, Specpure™, NIST Traceable CAS 1336-21-6View Details
1336-21-6 -
 Buffer solution pH 10.00 (+/-0.024 @ 25oC) No Color Specpure NIST Traceable CAS 1336-21-6View Details
Buffer solution pH 10.00 (+/-0.024 @ 25oC) No Color Specpure NIST Traceable CAS 1336-21-6View Details
1336-21-6 -
 Buffer solution pH 10.00 (+/-0.024 @ 25oC) No Color Specpure NIST Traceable CAS 1336-21-6View Details
Buffer solution pH 10.00 (+/-0.024 @ 25oC) No Color Specpure NIST Traceable CAS 1336-21-6View Details
1336-21-6 -
 Buffer solution pH 10.00 (+/-0.024 @ 25oC) No Color Specpure NIST Traceable CAS 1336-21-6View Details
Buffer solution pH 10.00 (+/-0.024 @ 25oC) No Color Specpure NIST Traceable CAS 1336-21-6View Details
1336-21-6 -
 Buffer solution pH 7.00 (+/-0.024 @ 25oC) Colored Green Specpure NIST Traceable CAS 1336-21-6View Details
Buffer solution pH 7.00 (+/-0.024 @ 25oC) Colored Green Specpure NIST Traceable CAS 1336-21-6View Details
1336-21-6 -
 Ammonium hydroxide CAS 1336-21-6View Details
Ammonium hydroxide CAS 1336-21-6View Details
1336-21-6 -
 Ammonium hydroxide CAS 1336-21-6View Details
Ammonium hydroxide CAS 1336-21-6View Details
1336-21-6
