Magnesium acetate
- CAS NO.:142-72-3
- Empirical Formula: C4H6MgO4
- Molecular Weight: 142.39
- MDL number: MFCD00003539
- EINECS: 205-554-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:31
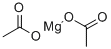
What is Magnesium acetate ?
Description
Anhydrous Magnesium Acetate has the chemical formula Mg(CH3COO)2 and in its hydrous form, Magnesium Acetate Tetrahydrate, it has the chemical formula Mg(CH3COO)2.4H2O. In this compound the magnesium metal has an oxidation state of 2+.

Magnesium acetate is the magnesium salt of acetic acid.It is deliquescent and upon heating, it decomposes to form magnesium oxide.Magnesium acetate is commonly used as a source of magnesium or as a chemical reagent.
Chemical properties
Anhydrous magnesium acetate, a white, crystalline, deliquescent solid, occurs in two forms: α-Mg(C2H3O2)2, formed by the reaction of MgO and concentrated acetic acid (13–33%) in boiling ethyl acetate, an and β-Mg(C2H3O2)2 which is formed using 5–6% acetic acid.
Physical properties
Magnesium acetate appears as white hygroscopic crystals. It smells like acetic acid and is soluble in water. When it is in an aqueous solution its pH can be considered to be neutral.
Characteristics
In 1881 Charles Clamond invented the Clamond basket, one of the first effective gas mantles. The reagents used in this invention included magnesium acetate, magnesium hydroxide and water.
Magnesium acetate is commonly used as a source of magnesium or for the acetate ion in chemistry experiments. One example of this is when magnesium acetate and magnesium nitrate were both used to perform molecular dynamics simulations and surface tension measurements. In the experiment the authors found that the acetate had a stronger affinity for the surface compared to the nitrate ion and that the Mg2+ strongly repelled away from the air/liquid interference. They also found that the Mg2+ had a stronger tendency to bind with the acetate ion compared to the nitrate.
One of the more prevalent uses of magnesium acetate is in the mixture called calcium magnesium acetate (CMA). It is a mixture of calcium acetate and magnesium acetate. CMA is thought of as an environmentally friendly alternative deicer to NaCl and CaCl2. CMA also acts as a powerful SO2, NOx, and toxic particulate emission control agent in coal combustion processes to reduce acid rain, and as an effective catalyst for the facilitation of coal combustion.
The Uses of Magnesium acetate
Dye fixative in textile printing, deodorant, disinfectant, and antiseptic.
Magnesium acetate is used in chemistry and molecular biology as a reagent and a source of magnesium. Magnesium has a variety of biological roles in enzymology, cell membrane and wall structural integrity, muscle cell physiology, and nucleic acid structure.Magnesium is an essential co-factor in many enzymes, including deoxyribonuclease (DNase), the restriction enzymes EcoR I and EcoR V, and Ribonuclease H. Magnesium also stabilizes polymeric nucleic acids such as transfer RNA and ribozymes.
Magnesium acetate has been widely used in the crystallization of proteins. A protocol for the separation of MB and BB isoenzymes of creatine kinase and the LD1 isoenzyme of lactate dehydrogenase that involves elution with magnesium acetate has been published.
The Uses of Magnesium acetate
The largest use for magnesium acetate is in the production of rayon fiber, which is used for cigarette filter tow. Magnesium acetate also has uses as a dye fixative in textile printing, as a deodorant, disinfectant, an antiseptic in medicine, and as a reagent chemical.
The Uses of Magnesium acetate
Magnesium acetate is used in molecular biology applications such as batch in vitro transcription of RNA and the crystallization of transcription factor:DNA complexes and proteins via the sitting-drop vapor-diffusion method. Also used as a buffer and to detect sodium and as a catalyst for sulfuric acid.
What are the applications of Application
Magnesium acetate solution is a biological buffer, ~1 M in H2O, pH 5.0-7.0 (25° C)
Definition
ChEBI: The magnesium salt of acetic acid.
Flammability and Explosibility
Non flammable
Safety
Magnesium Acetate is a relatively safe compound to handle and has been given a health hazard rating of zero. However, it should always be handled with gloves and safety goggles. If it is gets in the eyes, the skin, ingested, or inhaled it will cause irritation in the respective areas: eyes, skin, gastrointestinal system, and lungs.
Synthesis
Synthesis of magnesium acetate from the reaction of magnesium hydroxide with acetic acid.
2CH3COOH + Mg(OH)2 → (CH3COO)2Mg + 2H2O
Magnesium carbonate suspended in distilled water with 20 % acetic acid solution.
2CH3COOH + MgCO3 → Mg(CH3COO)2
Reacting metallic magnesium with acetic acid dissolved in dry nitrogen benzene causes Magnesium Acetate to form along with the release a gas, presumably hydrogen.
Mg +2CH3COOH → Mg(CH3COO)2 + H2.
Metabolism
Not Available
Storage
Due to the fact that it is very hygroscopic, it must be stored away from water. It is also incompatible with strong oxidizers and should not be mixed with them.
Purification Methods
Crystallise it from anhydrous acetic acid, then dry it under vacuum for 24hours at 100o. [Nencollas J Chem Soc 744 1956, Beilstein 2 IV 113.]
Properties of Magnesium acetate
| Melting point: | 72-75 °C(lit.) |
| Density | 1.5000 |
| refractive index | n |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Liquid |
| pka | 4.756[at 20 ℃] |
| color | white orthorhombic/monoclinic
crystals, crystalline |
| PH | 7.95(1 mM solution);8.23(10 mM solution);8.41(100 mM solution);8.78(1000 mM solution) |
| Water Solubility | Not miscible or difficult to mix with water. |
| λmax | λ: 260 nm Amax: 0.04 λ: 280 nm Amax: 0.02 |
| Merck | 13,5676 |
| BRN | 3692530 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Avoid moisture. |
| CAS DataBase Reference | 142-72-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Magnesium acetate(142-72-3) |
| EPA Substance Registry System | Magnesium acetate (142-72-3) |
Safety information for Magnesium acetate
Computed Descriptors for Magnesium acetate
| InChIKey | UEGPKNKPLBYCNK-UHFFFAOYSA-L |
Magnesium acetate manufacturer
Madan Minerals & Chemicals Industries
Krishna Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 MAGNESIUM ACETATE BP 99%View Details
MAGNESIUM ACETATE BP 99%View Details -
 Magnesium acetate 99%View Details
Magnesium acetate 99%View Details -
 Magnesium acetate 98%View Details
Magnesium acetate 98%View Details -
 Magnesium acetate anhydrousView Details
Magnesium acetate anhydrousView Details
142-72-3 -
 Magnesium Acetate powder, Grade: 98, Purity: 99View Details
Magnesium Acetate powder, Grade: 98, Purity: 99View Details
142-72-3 -
 Magnesium Acetate Chemical, For IndustrialView Details
Magnesium Acetate Chemical, For IndustrialView Details
142-72-3 -
 Solid Magnesium Acetate Anhydrous, 25/50 Kg, Packaging Type: BagView Details
Solid Magnesium Acetate Anhydrous, 25/50 Kg, Packaging Type: BagView Details
142-72-3 -
 Industrial Magnesium Acetate Anhydrous, Packaging Size: 25/50 kgView Details
Industrial Magnesium Acetate Anhydrous, Packaging Size: 25/50 kgView Details
142-72-3
