Cobalt acetate
Synonym(s):Cobalt diacetate;Cobaltous acetate;Cobaltous diacetate
- CAS NO.:71-48-7
- Empirical Formula: C4H6CoO4
- Molecular Weight: 177.02
- MDL number: MFCD00008689
- EINECS: 200-755-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
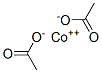
What is Cobalt acetate?
Chemical properties
Violet Crystalline Powder or Pale pink to purple Powder. Catalyzer for oxidizing dimethylbenzene, desiccant for coating, mordant for printing, accelerant for solidifying glass.

Cobalt(II) acetate is one of the compounds recommended for colouring the oxide layer formed on aluminium and its alloys by anodizing.
Physical properties
Red-to-violet monoclinic crystals (anhydrous acetate is light pink in color); density 1.705 g/cm3; becomes anhydrous when heated at 140°C; soluble in water, alcohols and acids.
The Uses of Cobalt acetate
Sympathetic inks, paint and varnish driers, catalyst, anodizing, mineral supplement in feed additives, foam stabilizer.
The Uses of Cobalt acetate
Cobalt(II) acetate is used as an industrial catalyst. It is used as a precursor to various oil drying agents. It finds application in ion exchange agents, lubricants, plating agents and surface treating agents, greases, ink, toner, and colorant products.
Definition
ChEBI: A cobalt salt in which the cobalt metal is in the +2 oxidation state and the counter-anion is acetate.
General Description
Red-violet crystalline solid. Vinegar-like odor.
Air & Water Reactions
Water soluble. Deliquescent
Reactivity Profile
Salts, basic, such as Cobalt acetate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
May not be used in food products (FDA).
Health Hazard
Inhalation causes shortness of breath and coughing; permanent disability may occur. Ingestion causes pain and vomiting. Contact with eyes causes irritation. Contact with skin may cause dermatitis.
Fire Hazard
Special Hazards of Combustion Products: Toxic cobalt oxide fumes may form in fire.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. Questionable carcinogen. Mutation data reported. See also COBALT COMPOUNDS. When heated to decomposition it emits acrid smoke and irritating fumes.
Properties of Cobalt acetate
| Melting point: | 298 °C (dec.)(lit.) |
| Density | 1.7043g/cm3 |
| solubility | Aqueous Acid (Slightly), Water (Slightly) |
| form | Powder |
| color | Pale pink to purple |
| Water Solubility | Soluble in waterSoluble in water, alcohol, dilute acids and pentyl acetate(tetrahydrate). |
| Sensitive | Hygroscopic |
| Merck | 14,2433 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 71-48-7(CAS DataBase Reference) |
| EPA Substance Registry System | Acetic acid, cobalt(2+) salt (2:1) (71-48-7) |
Safety information for Cobalt acetate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cobalt acetate
| InChIKey | QAHREYKOYSIQPH-UHFFFAOYSA-L |
Cobalt acetate manufacturer
Zama Chemical
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Cobalt Acetate 98%View Details
Cobalt Acetate 98%View Details -
 Cobalt(II) acetate 98%View Details
Cobalt(II) acetate 98%View Details -
 Cobalt(II) acetate 99%View Details
Cobalt(II) acetate 99%View Details -
 Cobalt(II) acetate, Anhydrous, Co 32% min. CAS 71-48-7View Details
Cobalt(II) acetate, Anhydrous, Co 32% min. CAS 71-48-7View Details
71-48-7 -
 Cobalt(II) acetate, Anhydrous, Co 32% min. CAS 71-48-7View Details
Cobalt(II) acetate, Anhydrous, Co 32% min. CAS 71-48-7View Details
71-48-7 -
 Cobalt Acetate PowderView Details
Cobalt Acetate PowderView Details
71-48-7 -
 Cobalt Acetate CrystalsView Details
Cobalt Acetate CrystalsView Details
6147-53-1 -
 Cobalt Acetate 500gmView Details
Cobalt Acetate 500gmView Details
6147-53-1
