Hydrogen bromide
Synonym(s):HBr;Hydrobromic acid;Hydrogen bromide in acetic acid;Hydrogen Bromide Solution, Hydrobromic Acid
- CAS NO.:10035-10-6
- Empirical Formula: BrH
- Molecular Weight: 80.91
- MDL number: MFCD00011323
- EINECS: 233-113-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-14 14:09:35
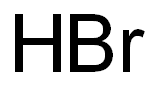
What is Hydrogen bromide?
Description
Hydrobromic Acid is a strong acid formed by dissolving the
diatomic molecule HBr in water. “Constant-boiling”
hydrobromic acid is an aqueous solution that distills at
124.3°C and contains 47.6% HBr by weight. Hydrobromic
acid has a pKa of 9, making it a stronger acid
than hydrochloric acid, but not as strong as HI, hydroiodic
acid. Hydrobromic acid is one of the strongest
mineral acids known.
Hydrobromic acid is mainly used for the production
of inorganic bromides, especially the bromides of zinc,
calcium, and sodium. It is a useful reagent for generating
organobromine compounds. Certain ethers are
cleaved with HBr. It also catalyzes alkylation reactions
and the extraction of certain ores. Industrially significant
organic compounds prepared from hydrobromic
acid include allyl bromide, tetrabromobis(phenol),
and bromoacetic acid.
Hydrobromic acid can be prepared in the laboratory
via the reaction of Br2, SO2 and water. Another laboratory
preparation involves the production of anhydrous
HBr, which is then dissolved in water.
Hydrobromic acid has commonly been prepared
industrially by reacting bromine with either sulfur or
phosphorous and water. However, it can also be
produced electrolytically. It can also be prepared by
treating bromides with nonoxidizing acids like phosphoric
or acetic acids. Hydrobromic acid is available
commercially in various concentrations and purities.
Description
Hydrobromic acid (HBr) is commonly sold as a 48% (by weight) solution in water. HBr works well as a Lewis acid catalyst but the increased nucleophilicity of bromide relative to chloride generally makes hydrochloric acid the preferable choice. However, in certain reactions such as in the deprotection of methyl ethers, the increased nucleophilicity of bromide is advantageous. HBr is also a reasonable choice in reactions where the presence of bromide already exists, such as Sandmeyer reactions and brominations.
Chemical properties
colourless liquid with a strong irritating odour
Physical properties
Colorless gas; fumes in moist air; pungent acrid odor; nonflammable; heav-ier than air; density 2.71 (air=1.0); gas density 3.55 g/L at 25°C; liquefies at-66.4°C; solidifies at -86.8°C; critical temperature 89.8°C; critical pressure84.5 atm; highly soluble in water (saturated aqueous solution contains 66%HBr at 25°C); forms a constant-boiling azeotrope at 47.5% HBr in solution,boiling at 126°C at atmospheric pressure; soluble in alcohol; a 0.10Maqueoussolution is 93% ionized to H+and Br ? ions at 18°C.
The Uses of Hydrogen bromide
Hydrogen bromide is used as a reagent and
catalyst in several types of organic reactions
such as the formation of alkyl bromides from
alcohols.
It is also used as a source material in the
preparation of inorganic bromides. Hydrogen
bromide serves as a catalyst in alkylation reactions.
It has also been reportedly used in the
controlled oxidation of aliphatic and alicyclic
hydrocarbons to peroxides, ketones, and acids.
In organic synthesis, hydrogen bromide is used
to substitute bromine for aliphatic chlorine in
the presence of aluminum catalyst.
The Uses of Hydrogen bromide

A mixture of the SM (2 g, 5.82 mmol), HBr in AcOH (12 mL), and aq HBr (6 mL) was heated at 100 C for 3 h. The reaction mixture was cooled to RT, extracted with EtOAc (2 x 100 mL), washed with brine, dried (Na2SO4), and concentrated to provide the product as an off-white solid. [1.3 g]
The Uses of Hydrogen bromide
The Concentrated acid is used principally in analytical chemistry and organic preparations.
The Uses of Hydrogen bromide
Hydrobromic acid is used in the manufacture of bromide, as an alkylation catalyst, and in organic synthesis.
Definition
Hydrogen bromide in aqueous solution.
Definition
A colorless liquid produced by adding hydrogen bromide to water. It shows the typical properties of a strong acid and it is a strong reducing agent. A convenient way of producing hydrobromic acid is to bubble hydrogen sulfide through bromine water. Although it is not as strong as hydrochloric acid it dissociates extensively in water and is a good proton donor.
Definition
hydrogen bromide: A colourlessgas, HBr; m.p. –88.5°C; b.p. –67°C. Itcan be made by direct combinationof the elements using a platinum catalyst.It is a strong acid dissociatingextensively in solution (hydrobromicacid).
Preparation
Hydrogen bromide gas may be produced by combustion of hydrogen inbromine vapor at 37.5°C using a catalyst such as platinized asbestos or pla-tinized silica gel. Unreacted free bromine is removed from the product bypassing the gaseous product mixture over hot activated charcoal. Hydrogenbromide formed may be absorbed in water to obtain the acid; or may be cooledand liquefied for shipment in cylinders.
Hydrobromic acid may be prepared in the laboratory by distillation of asolution of potassium bromide with dilute sulfuric acid:
2KBr + H2SO4 → K2SO4 + HBr
The acid may be prepared by several other methods, as well, including reac-tion of bromine either with sulfur and water; or with phosphorus and water:
2Br2 + S + 2H2O → 4HBr + SO2
Hydrobromic acid also may be prepared by hydrogen exchange with a sodiumor potassium bromide solution when the solution is passed through a cation-exchange resin.
Hydrobromic acid is stored and shipped in drums, tanks, carboys, or bot-tles, labeled as corrosive materials. The anhydrous gas is stored and shippedin cylinders under its vapor pressure.
General Description
Hydrobromic acid solution (HBr) is a clear, yellow or brown colored liquid. Its reaction with K has been studied by a molecular beam technique.
Air & Water Reactions
Acrid odor, fumes in moist air forming clouds containing hydrobromic acid. Heat of solution large, [Merck, 11th ed., 1989].
Reactivity Profile
HYDROGEN BROMIDE is an anhydrous (no water) strong acid. Reacts rapidly and exothermically with bases of all kinds (including amines and amides). Reacts exothermically with carbonates (including limestone and building materials containing limestone) and hydrogen carbonates to generate carbon dioxide. Reacts with sulfides, carbides, borides, and phosphides to generate toxic or flammable gases. Reacts with many metals (including aluminum, zinc, calcium, magnesium, iron, tin and all of the alkali metals) to generate flammable hydrogen gas. Reacts violently with acetic anhydride, 2-aminoethanol, ammonium hydroxide, calcium phosphide, chlorosulfonic acid, 1,1-difluoroethylene, ethylenediamine, ethyleneimine, oleum, perchloric acid, b-propiolactone, propylene oxide, silver perchlorate/carbon tetrachloride mixture, sodium hydroxide, uranium(IV) phosphide, vinyl acetate, calcium carbide, rubidium carbide, cesium acetylide, rubidium acetylide, magnesium boride, mercury(II) sulfate, calcium phosphide, calcium carbide.
Hazard
Toxic by inhalation, strong irritant to eyes and skin.
Health Hazard
Hydrobromic acid and hydrogen bromide gas are highly corrosive substances that
can cause severe burns upon contact with all body tissues. The aqueous acid and gas
are strong eye irritants and lacrimators. Contact of concentrated hydrobromic acid or
concentrated HBr vapor with the eyes may cause severe injury, resulting in
permanent impairment of vision and possible blindness. Skin contact with the acid
or HBr gas can produce severe burns. Ingestion can lead to severe burns of the
mouth, throat, and gastrointestinal system and can be fatal. Inhalation of hydrogen
bromide gas can cause extreme irritation and injury to the upper respiratory tract and
lungs, and exposure to high concentrations may cause death. HBr gas is regarded as
having adequate warning properties.
Hydrogen bromide has not been found to be carcinogenic or to show reproductive or
developmental toxicity in humans.
Health Hazard
Hydrobromic acid is a corrosive liquid. Thegas is a strong irritant to the eyes, nose, andmucous membranes. In humans, exposure to5 ppm for a few minutes can cause irritationof the nose. Irritation of the eyes and lungsmay be felt at higher concentrations. Thedetectable odor threshold is 2 ppm.
Fire Hazard
Behavior in Fire: Pressurized container may explode and release toxic, irritating vapor.
Flammability and Explosibility
Noncombustible, but contact with metals may produce highly flammable hydrogen gas.
Materials Uses
Hydrogen bromide does not aggressively attack common metals of construction while in the anhydrous state. However, in the presence of moisture, hydrogen bromide will attack most metals except platinum and silver. Galvanized pipe, brass, and bronze should be avoided. Steel, Monel, and aluminum-silicon-bronze have proven satisfactory in anhydrous hydrogen bromide service.
Potential Exposure
Hydrogen bromide gas and its aqueoussolutions are used in the manufacture of organic and inor-ganic bromides; as a reducing agent and catalyst in con-trolled oxidations; in the alkylation of aromatic compounds;and in the isomerization of conjugated diolefins. It is usedas an intermediate for pharmaceuticals, dyes, photographicchemicals.
Physiological effects
Hydrogen bromide is extremely irritating to the eyes, mucous membranes of the respiratory tract, and skin. Contact may cause bums. Repeated short exposures to concentrations of about 35 ppm can cause irritation to the nose and throat with mucous production and indigestion. Inhalation of higher concentrations can cause pulmonary edema and laryngeal spasm, and can be fatal. Skin contact with the vapor or liquid causes severe tissue irritation and necrosis [2].
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek med-ical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, includ-ing resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medi-cal attention. If victim is conscious, administer water ormilk. Do not induce vomiting. Medical observation isrecommended for 24- 48 h after breathing overexposure, aspulmonary edema may be delayed. As first aid for pulmo-nary edema, a doctor or authorized paramedic may consideradministeringa corticosteroid spray. If frostbite hasoccurred, seek medical attention immediately; do NOT rubthe affected areas or flush them with water. In order to pre-vent further tissue damage, do NOT attempt to remove fro-zen clothing from frostbitten areas. If frostbite has NOToccurred, immediately and thoroughly wash contaminatedskin with soap and water.
storage
Splash goggles and rubber gloves should be
worn when handling this acid, and containers of HBr should be stored in a wellventilated
location separated from incompatible metals. Water should never be
added to HBr because splattering may result; always add acid to water. Containers of
hydrobromic acid should be stored in secondary plastic trays to avoid corrosion of
metal storage shelves due to drips or spills.
Cylinders of hydrogen bromide
should be stored in cool, dry locations, separated from alkali metals and other
incompatible substances.
Shipping
Anhydrous hydrogen bromide requires a shippinglabel of“POISON GAS, CORROSIVE."It falls in HazardClass 2.3. It is a violation of transportation regulations torefill compressed gas cylinders without the express writtenpermission of the owner.Special precautions:Cylinders must be transported in asecure upright position, in a well-ventilated truck.Hydrobromic acid, with > 49% hydrobromic acid requires ashipping label of“CORROSIVE." It falls in Hazard Class 8and Packing GroupII or II.Hydrobromic acid,with not > 49% hydrobromic acidrequires a shipping label of“CORROSIVE.”It falls inHazard Class 8 and Packing GroupII or II.
Purification Methods
A solution of aqueous HBr ca 48% (w/w, constant boiling) is purified by distilling twice with a little red phosphorus, and the middle half of the distillate is taken. (The azeotrope at 760mm contains 47.8% (w/w) HBr.) [Hetzer et al. J Phys Chem 66 1423 1962]. Free bromine can be removed by Irvine and Wilson's method for HI (see above), except that the column is regenerated by washing with an ethanolic solution of aniline or styrene. Hydrobromic acid can also be purified by aerating with H2S, distilling and collecting the fraction boiling at 125-127o. [Heisig & Andur Inorg Synth I 155 1939.] HARMFUL VAPOURS.
Incompatibilities
Hydrobromic acid and hydrogen bromide react violently with many metals with the generation of highly flammable hydrogen gas, which may explode. Reaction with oxidizers such as permanganates, chlorates, chlorites, and hypochlorites may produce chlorine or bromine.
Waste Disposal
In many localities, hydrobromic acid or the residue from a spill may be disposed of down the drain after appropriate dilution and neutralization. Otherwise, hydrobromic acid and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. Excess hydrogen bromide in cylinders should be returned to the manufacturer. For more information on disposal procedures, see Chapter 7 of this volume.
GRADES AVAILABLE
Hydrogen bromide is typically available in 99.8
percent purity.
Gas purity guidelines have been developed
and published by Semiconductor Equipment
and Materials International and can be found in
the Book of SEMI Standards, Gases Volume.
Properties of Hydrogen bromide
| Melting point: | −87 °C(lit.) |
| Boiling point: | −67 °C(lit.) |
| Density | 1.49 g/mL at 25 °C (lit.) |
| vapor density | 2.8 (vs air) |
| vapor pressure | 334.7 psi ( 21 °C) |
| refractive index | n |
| Flash point: | 40°C |
| storage temp. | Store below +30°C. |
| solubility | soluble |
| appearance | Colorless to light yellow liquid (48% w/w aq.) |
| form | Solution |
| pka | -9(at 25℃) |
| Specific Gravity | 1.49 |
| color | Light yellow, brown |
| Odor | Sharp, irritating odor detectable at 2 ppm |
| PH | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,4778 |
| BRN | 3587158 |
| Exposure limits | Ceiling limit 3 ppm (~10 mg/m3) (ACGIH);
TLV-TWA 3 ppm (~10 mg/m3) (MSHA and
OSHA). |
| Dielectric constant | 7.0(-85℃) |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents, ammonia, ozone, fluorine, water, metals. Air and light sensitive. |
| CAS DataBase Reference | 10035-10-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydrogen bromide(10035-10-6) |
| EPA Substance Registry System | Hydrobromic acid (10035-10-6) |
Safety information for Hydrogen bromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Hydrogen bromide
Abamectin manufacturer
Sagar Speciality Chemicals Private Limited
Pallav Chemicals And Solvents Pvt Ltd
ARRAKIS INDUSTRIES LLP
SS Reagents and Chemicals
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 Hydrobromic acid CAS 10035-10-6View Details
Hydrobromic acid CAS 10035-10-6View Details
10035-10-6 -
 Hydrobromic acid CAS 10035-10-6View Details
Hydrobromic acid CAS 10035-10-6View Details
10035-10-6 -
 Hydrobromic acid CAS 10035-10-6View Details
Hydrobromic acid CAS 10035-10-6View Details
10035-10-6 -
 Hydrobromic acid CAS 10035-10-6View Details
Hydrobromic acid CAS 10035-10-6View Details
10035-10-6 -
 Hydrobromic acid 47% CASView Details
Hydrobromic acid 47% CASView Details -
 Hydrobromic Acid 48% CASView Details
Hydrobromic Acid 48% CASView Details -
 Hydrobromic acid 47% CASView Details
Hydrobromic acid 47% CASView Details -
 Hydrobromic acid 47% CASView Details
Hydrobromic acid 47% CASView Details