Cuprous bromide
Synonym(s):Cuprous bromide
- CAS NO.:7787-70-4
- Empirical Formula: BrCu
- Molecular Weight: 143.45
- MDL number: MFCD00010969
- EINECS: 232-131-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-18 19:10:02
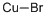
What is Cuprous bromide?
Chemical properties
Copper(I) bromide is a white, cubic, crystalline material that decomposes on exposure to light or moisture in air. It forms complexes with hydrochloric and hydrobromic acids and aqueous ammonia.
The Uses of Cuprous bromide
As catalyst for organic reactions.
The Uses of Cuprous bromide

To ACN (350 mL) at RT was added CuBr2 (67 g, 300 mmol). The mixture was cooled to 0-5 C and was treated dropwise with t-BuONO (72.5 mL, 535 mmol). The mixture was stirred for 5 min, then was added the SM (35 g, 238 mmol). The reaction was stirred at RT for 2 h. EtOAc (525 mL) was added to the mixture and the reaction was quenched with 10% aq NH4Cl (350 mL) and 2% aq NH3 (175 mL). The layers were separated and the aq layer was extracted with EtOAc. The combined organics were dried and concentrated. The resulting material was purified by silica gel chromatography (eluting with 5% EtOAc/hexane) to provide the product as a yellow solid. [35 g, 70%]
The Uses of Cuprous bromide
Copper bromide is also known as cupric bromide, this substance was made by double decomposition when mixing aqueous solutions of copper sulfate and potassium bromide. This greenish blue solution was used as the bleaching step for intensifying collodion and gelatin negatives.
The Uses of Cuprous bromide
It is used as effective catalyst for tetrahydropyranylation of alcohols. It can also be used in combination with palladium in catalytic synthesis of 3-haloindoles through annulation process. It is widely used in the synthesis of organic fine chemicals. The compound is widely used in the synthesis of organic compounds. It is also useful for copper-mediated coupling reactions. A silica gel support copper bromide hexa methyltriethylenetetramine complex has shown useful application for the atom transfer radical polymerization of methyl methacrylate in toluene.
Preparation
It is prepared pyrometallurgically with copper metal and elemental bromine in a process similar to that for copper(I) chloride. It can also be produced by the reduction of copper(II) sulfate solutions in the presence of sodium bromide using metallic copper or sulfite as the reducing agent. Copper(I) bromide is used as a polymerization catalyst in organic reactions.
General Description
White powder or crystal. Turns green to dark blue on exposure to sunlight. Sinks and mixes slowly with water.
Air & Water Reactions
Mixes slowly with water.
Reactivity Profile
Cuprous bromide has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.
Health Hazard
INHALATION: Irritation of upper respiratory tract. EYES: Irritation of conjunctivae. SKIN: Irritation, acne-like rash (usually from prolonged exposure). INGESTION: Vomiting caused by local irritant and astringent action of ionic Cu on stomach and intestines. Pain in mouth, esophagus, and stomach. Inorganic bromides produce depression, psychoses, and mental deterioration.
Purification Methods
Purify it as for cuprous iodide but using aqueous NaBr. [Keller & Wycoff Inorg Synth II 3 1946, Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1006 1965.]
Properties of Cuprous bromide
| Melting point: | 504 °C(lit.) |
| Boiling point: | 1345 °C |
| Density | 4.71 g/mL at 25 °C(lit.) |
| Flash point: | 1345°C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | insoluble in acetone |
| appearance | White solid (pure) |
| form | Powder |
| color | Pale green to green |
| Specific Gravity | 4.98 |
| Water Solubility | Soluble in hydrogen bromide, hydrochloric acid, and ammonium hydroxide. Slightly soluble in water. Insoluble in acetone. |
| Sensitive | Air Sensitive |
| Crystal Structure | Hexagonal, Wurtzite (Zincite) Structure - Space Group P 63mc |
| Merck | 14,2659 |
| Solubility Product Constant (Ksp) | pKsp: 8.2 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Stability: | Stable, but may be air or light sensitive. Incompatible with alkali metals, strong oxidizing agents. |
| CAS DataBase Reference | 7787-70-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Copper(I) bromide(7787-70-4) |
| EPA Substance Registry System | Copper bromide (CuBr) (7787-70-4) |
Safety information for Cuprous bromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cuprous bromide
| InChIKey | NKNDPYCGAZPOFS-UHFFFAOYSA-M |
Cuprous bromide manufacturer
JSK Chemicals
Zama Chemical
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Copper Bromide 98%View Details
Copper Bromide 98%View Details -
 Copper(I) bromide CAS 7787-70-4View Details
Copper(I) bromide CAS 7787-70-4View Details
7787-70-4 -
 Copper bromide CAS 7787-70-4View Details
Copper bromide CAS 7787-70-4View Details
7787-70-4 -
 Copper(I) bromide CAS 7787-70-4View Details
Copper(I) bromide CAS 7787-70-4View Details
7787-70-4 -
 Copper(I) bromide CAS 7787-70-4View Details
Copper(I) bromide CAS 7787-70-4View Details
7787-70-4 -
 Copper(I) bromide CAS 7787-70-4View Details
Copper(I) bromide CAS 7787-70-4View Details
7787-70-4 -
 Copper(I) Bromide (CuBr)View Details
Copper(I) Bromide (CuBr)View Details
7787-70-4 -
 Cuprous BromideView Details
Cuprous BromideView Details
7787-70-4
