Lead(II) nitrate
Synonym(s):Lead dinitrate;Lead(II) nitrate
- CAS NO.:10099-74-8
- Empirical Formula: N2O6Pb
- Molecular Weight: 331.21
- MDL number: MFCD00011153
- EINECS: 233-245-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
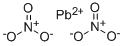
What is Lead(II) nitrate?
Chemical properties
The lead nitrate appears as a white cubic crystal or monoclinic crystalline powder. It is soluble in water and liquid ammonia, being slightly soluble in the ethanol, being insoluble in concentrated nitric acid (produce protective film); it can react with concentrated hydrochloric acid or concentrated alkali chloride solution to form complex chlorinated lead acid or chlorinated lead acid salt; it has strong oxidizing property and may cause the risk of inducing combustion and explosion if mixed with organic matter, reducing agent and combustible substance such as sulfur and phosphorus or even subject to slightly friction. The dry lead nitrate can be subject to decomposition at around 205 ~223 while a temperature of 100 is enough to trigger the decomposition of wet lead nitrate. Its decomposition can emit toxic nitric oxide gas.
Chemical properties
Lead nitrate, Pb(NO3)2, sp gr 4.53, forms cubic or monoclinic colorless crystals. Above 205 °C, oxygen and nitrogen dioxide are driven off, and basic lead nitrates are formed. Above 470 °C, lead nitrate is decomposed to lead monoxide and Pb3O4. Lead nitrate is highly soluble in water (56.5 g/100 mL at 20 °C; 127 g/100 mL at 100 °C), soluble in alkalies and ammonia, and fairly soluble in alcohol (8.77 g/ 100 mL of 43% aqueous ethanol at 22 °C). Lead nitrate is readily obtained by dissolving metallic lead, lead monoxide, or lead carbonate in dilute nitric acid. Excess acid prevents the formation of basic nitrates, and the desired lead nitrate can be crystallized by evaporation.
Physical properties
Colorless cubic or monoclinic crystals; refractive index 1.782; density 4.53 g/cm3 at 20°C; decomposes at 470°C; soluble in cold water; very soluble in boiling water 127 g/100 mL at 100°C; also soluble in caustic soda, caustic potash and ammonia solution, and moderately soluble in alcohol.
The Uses of Lead(II) nitrate
Lead(II) nitrate is employed in industrial applications such as heat stabilization in nylon and polyesters and coatings of photothermographic paper. It is utilized to improve the leaching process in the gold cyanidation. It finds applications as a lead paint as well as a source of dinitrogen tetroxide. As an oxidant, it is used in the oxidation of benzylic halides to aldehydes in organic synthesis. It is also used in the preparation of dithiocarbamates from isothiocyanates. It acts as a scavenger of bromide in nucleophilic substitution reactions.
The Uses of Lead(II) nitrate
manufacture of matches and special explosives; as mordant in dyeing and printing on textiles; mordant for staining horn, mother-of-pearl; oxidizer in dye industry; sensitizer in photography; process engraving.
The Uses of Lead(II) nitrate
Lead nitrate is used in many industrial processes, ranging from ore processing to pyrotechnics to photothermography. Thus lead nitrate is used as a flotation agent in titanium removal from clays; in electrolytic refining of lead; in rayon delustering; in red lead manufacture; in matches, pyrotechnics, and explosives; as a heat stabilizer in nylon; as a coating on paper for photothermography; as an esterification catalyst for polyesters; as a rodenticide; as an electroluminescent mixture with zinc sulfide; as a means of electrodepositing lead dioxide coatings on nickel anodes; and as a means of recovering precious metals from cyanide solutions.
What are the applications of Application
Lead (II) nitrate is Titrant for complexometric titration
Preparation
Lead nitrate is prepared by dissolving lead metal, lead monoxide or lead carbonate in excess dilute nitric acid followed by evaporation of and/or cooling the solution for crystallization.
General Description
Lead dinitrate is a white crystalline solid. The material is soluble in water. Lead dinitrate is noncombustible but Lead dinitrate will accelerate the burning of combustible materials. If large quantities of the material are involved in the fire an explosion may result. Prolonged exposure of the material to fire or heat may result in an explosion. Toxic oxides of nitrogen are produced in fires involving Lead dinitrate.
Air & Water Reactions
Water soluble.
Reactivity Profile
Mixtures of metal/nonmetal nitrates with alkyl esters may explode because of the formation of alkyl nitrates; mixtures of nitrate with phosphorus, tin (II) chloride or other reducing agents may react explosively [Bretherick 1979. p. 108-109]. An explosion of guanidine nitrate demolished an autoclave built to withstand 50 atmospheres, in which Lead dinitrate was being made from ammonium thiocyanate and Lead dinitrate [C. Angew. Chem. 49:23. 1936].
Hazard
The toxic effects are greater than other lead salts because lead nitrate is more soluble. Moderately toxic by ingestion and other routes of exposure. The compound also is an irritant to eye, skin, and mucous membranes.
Health Hazard
Early symptoms of lead intoxicatin via inhalation or ingestion are most commonly gastrointestinal disorders, colic, constipation, etc.; weakness, which may go on to paralysis, chiefly of the extensor muscles of the wrists and less often the ankles, is noticeable in the most serious cases. Ingestion of a large amount causes local irritation of the alimentary tract; pain, leg cramps, muscle weakness, paresthesias, depression, coma, and death may follow in 1 or 2 days. Contact with eyes causes irritation.
Flammability and Explosibility
Non flammable
Industrial uses
This is a white to colorless fine crystalline compound, extremely soluble in water (34% at 20 °C). Commercial production is based on dissolution of lead metal or lead compounds in nitric acid (36–40% solution). Lead nitrate is considered to be an activator in mineral processing. Although lead may activate sphalerite, similar to CuSO4, the use of Pb(NO3)2 is limited to the activation of stibnite during beneficiation of antimony ores. Lead nitrate is the most widely used chemical in cyanidation of precious metals as an accelerator.
Purification Methods
Precipitate it twice from a hot (60o) concentrated aqueous solution by adding HNO3. The precipitate is sucked dry on a sintered-glass funnel, then transferred to a crystallising dish which is covered by a clock glass and left in an electric oven at 110o for several hours [Beck et al. Trans Faraday Soc 55 331 1959]. After two recrystallisations of ACS grade, no metals above 0.001ppm were detected.
Properties of Lead(II) nitrate
| Melting point: | 470 °C (dec.)(lit.) |
| Boiling point: | 500℃[at 101 325 Pa] |
| Density | 1.00 g/mL at 20 °C |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble |
| form | Solid |
| color | White |
| Specific Gravity | 4.53 |
| PH | 3-4 (50g/l, H2O, 20℃) |
| Water Solubility | 343 g/L |
| Sensitive | Hygroscopic |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| Merck | 14,5414 |
| Exposure limits | ACGIH: TWA 0.05 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 0.050 mg/m3 |
| Dielectric constant | 37.7(0.0℃) |
| Stability: | Stable. Strong oxidizer. Incompatible with combustible materials, organics, strong reducing agents. |
| CAS DataBase Reference | 10099-74-8(CAS DataBase Reference) |
| EPA Substance Registry System | Lead nitrate (Pb(NO3)2) (10099-74-8) |
Safety information for Lead(II) nitrate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Lead(II) nitrate
Lead(II) nitrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Lead(II) nitrate, For ACS analysis CAS 10099-74-8View Details
Lead(II) nitrate, For ACS analysis CAS 10099-74-8View Details
10099-74-8 -
 Lead(II) nitrate, For ACS analysis CAS 10099-74-8View Details
Lead(II) nitrate, For ACS analysis CAS 10099-74-8View Details
10099-74-8 -
 Lead(II) nitrate CAS 10099-74-8View Details
Lead(II) nitrate CAS 10099-74-8View Details
10099-74-8 -
 Lead(II) nitrate CAS 10099-74-8View Details
Lead(II) nitrate CAS 10099-74-8View Details
10099-74-8 -
 LEAD NITRATE 99.5% AR, 500gm BottleView Details
LEAD NITRATE 99.5% AR, 500gm BottleView Details
10099-74-8 -
 Lead Nitrate, >99%, 25Kg BagView Details
Lead Nitrate, >99%, 25Kg BagView Details
10099-74-8 -
 Lead (II) nitrateView Details
Lead (II) nitrateView Details
10099-74-8 -
 Powder Lead NitrateView Details
Powder Lead NitrateView Details
10099-74-8
