LasMiditan
- CAS NO.:439239-90-4
- Empirical Formula: C19H18F3N3O2
- Molecular Weight: 377.36
- MDL number: MFCD18633238
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-01-08 15:16:02
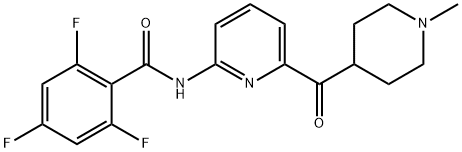
What is LasMiditan?
Absorption
Oral absorption of lasmiditan is quick, with a median tmax of 1.8 hours. An open-label study looking at absorption pharmacokinetics found the Cmax and AUC0-t of lasmiditan following oral administration to be 322.8 ± 122.0 ng/mL and 1892 ± 746.0 ng.h/mL, respectively. The oral bioavailability of lasmiditan has been reported as approximately 40%.
Co-administration of lasmiditan with a high-fat meal increased its Cmax and AUC by 22% and 19%, respectively, and delayed Tmax by approximately 1 hour - these differences in absorption are relatively minor and unlikely to be clinically significant. Similarly, severe renal impairment and mild-moderate hepatic impairment were found to increase both AUC and Cmax, but not to a clinically significant extent.
Toxicity
Data regarding overdose of lasmiditan is currently unavailable. Non-clinical murine toxicology studies revealed no evidence of carcinogenesis, mutagenesis, or impairment of fertility at plasma concentrations well above those seen in humans.
Description
Lasmiditan (Item No. 28369) is an analytical reference standard categorized as an agonist of the serotonin (5-HT) receptor subtype 5-HT1F. Formulations containing lasmiditan have been used in the treatment of migraine headache. Lasmiditan has low abuse potential. Lasmiditan is regulated as a Schedule V compound in the United States. This product is intended for research and forensic applications.
The Uses of LasMiditan
Lasmiditan is used in diagnosing, treating and/or preventing periodontal disease and/or tooth loss.
Background
Lasmiditan is an oral medication used in the termination of migraine headaches that was first approved for use in the United States in October 2019. It was also approved by the European Commission on August 17, 2022.
Traditionally, the triptan class of anti-migraine medications (e.g. sumatriptan) have seen preferential use in the acute treatment of migraines due to their relatively favourable efficacy and safety. Their use is not devoid of concerns, however, and their vasoconstrictive activity can lead to blood pressure lability and other cardiovascular side effects - for this reason, these medications are less suitable for use in patients with pre-existing cardiovascular disorders. Triptans abort migraines via action at several serotonin receptors, including 5-HT1D and 5-HT1B receptors, and activity at the 5-HT1B receptor has been specifically implicated in their vasoconstrictive activity.
Lasmiditan, in contrast, is a highly selective agonist of 5-HT1F receptors, carrying virtually no affinity for other receptors which appear to be largely responsible for the adverse effect profile of its predecessors - in other words, lasmiditan’s selectivity allows for the successful termination of migraines without causing vasoconstriction. Selectivity for 5-HT1F, a lack of vasoconstrictive activity, and the ability to terminate migraines through neuronal inhibition has resulted in the creation of a new class of anti-migraine medications in which lasmiditan is the first and only member: the neurally-acting anti-migraine medications (NAAMAs).
Indications
Lasmiditan is indicated for the acute treatment of migraine with or without aura in adults.
Pharmacokinetics
Lasmiditan belongs to a new and novel class of acute anti-migraine medications that exert their effects via inhibition of neuronal firing rather than vasoconstriction of cerebral arteries. Lasmiditan appears to have a relatively quick onset of action (an important characteristic in acute migraine treatment) with some patients reporting benefit within 20 minutes. Due to its ability to cause CNS depression (e.g. drowsiness, dizziness), lasmiditan may cause significant driving impairment and patients should be advised not to participate in activities requiring mental alertness for at least 8 hours after dosing. Lasmiditan may carry some potential for abuse and should be used with caution in patients who may be at risk of drug abuse - its controlled substance scheduling is currently under review in the United States by the Drug Enforcement Administration (DEA).
The safety of lasmiditan in pregnancy is unknown and is currently being monitored with a pregnancy exposure registry created by Eli Lilly and Company.
Metabolism
The hepatic and extra-hepatic metabolism of lasmiditan is catalyzed primarily by non-CYP enzymes, with ketone reduction appearing to be the primary pathway. While the specific enzymes involved in the metabolism of lasmiditan have not been elucidated, FDA labeling states that the following enzymes are not involved in its metabolism: monoamine oxidases, CYP450 reductase, xanthine oxidase, alcohol dehydrogenase, aldehyde dehydrogenase, and aldo-keto reductases. The metabolites of lasmiditan have not been characterized in published research, but two of its metabolites (M7 and M18) are considered to be pharmacologically inactive.
Properties of LasMiditan
| Melting point: | 255℃ |
| Boiling point: | 433.3±45.0 °C(Predicted) |
| Density | 1.351 |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), DMSO (Slightly) |
| form | Solid |
| pka | 9.91±0.70(Predicted) |
| color | White to Off-White |
Safety information for LasMiditan
Computed Descriptors for LasMiditan
Abamectin manufacturer
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 Lasmiditan 98%View Details
Lasmiditan 98%View Details -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4