Anacetrapib
- CAS NO.:875446-37-0
- Empirical Formula: C30H25F10NO3
- Molecular Weight: 637.51
- MDL number: MFCD16294903
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
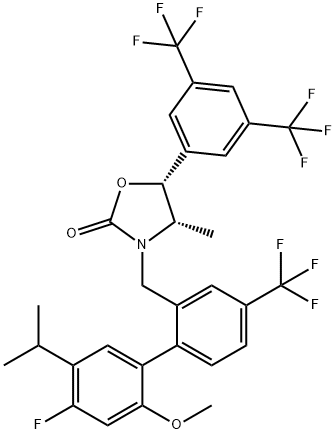
What is Anacetrapib?
Description
The “trapib” family of drugs are cholesterylester transfer protein (CETP) inhibitors that increase high-density lipoproteins (HDLs) and decrease low-density lipoproteins (LDLs) in the body. The molecules typically contain aromatic rings, nitrogen heterocycles, and multiple trifluoromethyl groups.
The first CETP inhibitor, torcetrapib, was developed by Pfizer and was discontinued because of adverse cardiac side effects. Two more recent ones, anacetrapib (Merck) and evacetrapib (Lilly), showed more promise, but they too are having tough sledding.
Anacetrapib has been in Phase II and III trials for more than a decade. Of some concern is that it remains in patients’ bodies for as long as 4 years. In 2013, however, Merck launched a 30,000-participant, double-blind, placebo-controlled Phase III study of anacetrapib that will continue through 2017.
Lilly, on the other hand, has?pulled the plug on evacetrapib. It did so in late 2015 after a 12,000-participant study showed that the drug did not reduce cardiovascular events compared with the placebo. The failure caused Lilly to take a $90 million charge against its fourth-quarter earnings.
The Uses of Anacetrapib
An orally active and potent cholesterol ester transfer protein (CETP) inhibitor for the treatment of atherosclerosis, in particular dyslipidemia.
What are the applications of Application
Anacetrapib is a potent and selective CETP inhibitor
Biological Activity
anacetrapib, also known as mk-0859, is a potent and selective inhibitor of cholesteryl ester transfer protein (cetp) that exhibits strong inhibition against cetp-mediated transfer of cholesteryl esters and triglycerides with values of inhibition constant ic50 of 16 nm and 29 nm respectively. anacetrapib is mainly metabolized by cyp3a4 through o-demethylation, hydroxylation on the biphenyl moiety and hydroxylation on the isopropyl side chain resulting in three oxidative metabolites m1, m2 and m3 respectively. anacetrapib is being investigated for the treatment of primary hypercholesterolemia and mixed hyperlipidemia due to its impressive effects to lower low-density lipoprotein (ldl) cholesterol levels and increase high-density lipoprotein (hdl) cholesterol levels without any associated major adverse events.tan ey, hartmann g, chen q, pereira a, bradley s, doss g, zhang as, ho jz, braun mp, dean dc, tang w, kumar s. pharmacokinetics, metabolism, and excretion of anacetrapib, a novel inhibitor of the cholesteryl ester transfer protein, in rats and rhesus monkeys. drug metab dispos. 2010;38(3):459-473.kumar s, tan ey, hartmann g, biddle z, bergman aj, dru j, ho jz, jones an, staskiewicz sj, braun mp, karanam b, dean dc, gendrano in, graves mw, wagner ja, krishna r. metabolism and excretion of anacetrapib, a novel inhibitor of the cholesteryl ester transfer protein, in humans. drug metab dispos. 2010;38(3):474-483
Enzyme inhibitor
This cholesteryl ester transfer protein (or CETP) inhibitor (FW = 637.51 g/mol; CAS 875446-37-0), also known by the code name MK-0859, reduces plasma cholesterol concentrations, thereby increasing serum highdensity lipoprotein levels and preventing cardiovascular disease associated with hypercholesteremia. X-ray crystal studies suggest that CETP is a crescent-shaped protein with a 6-nm hydrophobic tunnel traversing its core Both of the tunnel’s two entrances face the protein’s concave surface, which most likely is the interaction platform for binding HDL particles and engaging its lipoprotein constituents. CETP mediates the reciprocal transfer of neutral lipids (e.g., cholesteryl esters and triglycerides) and phospholipids between different lipoprotein fractions in blood plasma. Anacetrapib is unlike many CETP inhibitors, which increase systolic blood pressure. Anacetrapib also exhibits a low-to-moderate degree of absorption after oral dosing and majority of the absorbed dose is eliminated via oxidation to a series of hydroxylated metabolites that undergo conjugation with glucuronate before excretion into bile. Unlike torcetrapib, another CETP inhibitor, anacetrapib does not elevate blood pressure, alter electrolytes, or cause any significant side effects.
Properties of Anacetrapib
| Boiling point: | 555.3±50.0 °C(Predicted) |
| Density | 1.345 |
| storage temp. | Store at -20°C |
| solubility | ≥31.9 mg/mL in DMSO; insoluble in H2O; ≥86.6 mg/mL in EtOH |
| form | solid |
| pka | -2.30±0.60(Predicted) |
| color | White to off-white |
Safety information for Anacetrapib
Computed Descriptors for Anacetrapib
Anacetrapib manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![5-Fluoro-2-[[(1S)-1-(4-fluorophenyl)ethyl]amino]-6-[(5-methyl-1H-pyrazol-3-yl)amino]-3-pyridinecarbonitrile](https://img.chemicalbook.in/CAS2/GIF/905586-69-8.gif)


You may like
-
 Anacetrapib 98% (HPLC) CAS 875446-37-0View Details
Anacetrapib 98% (HPLC) CAS 875446-37-0View Details
875446-37-0 -
 Anacetrapib 98% CAS 875446-37-0View Details
Anacetrapib 98% CAS 875446-37-0View Details
875446-37-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
