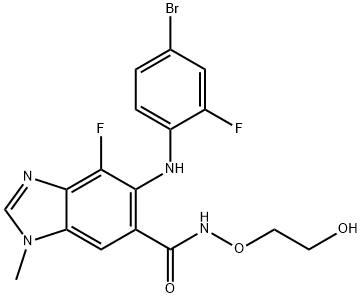
Binimetinib
- CAS NO.:606143-89-9
- Empirical Formula: C17H15BrF2N4O3
- Molecular Weight: 441.23
- MDL number: MFCD22124525
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is Binimetinib?
Absorption
The pharmacokinetics of binimetinib was studied in healthy subjects and patients with solid tumors. After twice-daily dosing, the accumulation is 1.5-fold and the coefficient of variation (CV%) of the area under the concentration-time curve (AUC) is <40% at steady state. The systemic exposure of binimetinib is approximately dose proportional.
After oral administration, at least 50% of the binimetinib dose was absorbed with a median time to maximum concentration (Tmax) of 1.6 hours.
The administration of a single dose of binimetinib 45 mg with a high-fat, high-calorie meal (consisting of approximately 150 calories from protein, 350 calories from carbohydrate, and 500 calories from fat) in healthy subjects had no effect on binimetinib exposure.
Toxicity
Based on findings from animal reproduction studies and its mechanism of action, binimetinib can cause fetal harm when administered to a pregnant woman. There are no available clinical data on the use of binimetinib during pregnancy. In animal reproduction studies, oral administration of binimetinib during the period of organogenesis was embryotoxic and an abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 5 times the human exposure at the clinical dose of 45 mg twice daily. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
No overall differences in the safety or effectiveness of MEKTOVI plus encorafenib were observed in older patients as compared to younger patients.
Since binimetinib is 97% bound to plasma proteins, hemodialysis is likely to be ineffective in the treatment of overdose with binimetinib.
Carcinogenicity studies with binimetinib have not been conducted. Binimetinib was not genotoxic in studies evaluating reverse mutations in bacteria, chromosomal aberrations in mammalian cells, or micronuclei in the bone marrow of rats.
Description
Binimetinib (606143-89-9) is a potent (IC50?= 12 nM) and selective allosteric inhibitor of MEK1/2.1,2?Recently approved by the FDA for treatment of melanoma in combination with Encorafenib. Binimetinib has had limited success as monotherapy but has shown promise in combination with other chemotherapeutic agents.3-5
The Uses of Binimetinib
MEK 162 is a MEK1/2 inhibitor allowing it to be a effective anti-cancer medication.
Background
Binimetinib, also known as Mektovi, is a potent and selective oral mitogen-activated protein kinase 1/2 (MEK 1/2) inhibitor which is combined with Encorafenib.
On June 27, 2018, the Food and Drug Administration approved the combination of Encorafenib and binimetinib (BRAFTOVI and MEKTOVI, from Array BioPharma Inc.) in combination for patients with unresectable or metastatic melanoma with the BRAF V600E or V600K mutations, as detected by an FDA-approved test.
Indications
Binimetinib, in conjunction with encorafenib, is indicated for the treatment of unresectable or metastatic melanoma with BRAF V600E or V600K mutation and metastatic non-small cell lung cancer (NSCLC) with a BRAF V600E mutation.
Definition
ChEBI: Binimetinib is a member of the class of benzimidazoles that is 1-methyl-1H-benzimidazole which is substituted at positions 4, 5, and 6 by fluorine, (4-bromo-2-fluorophenyl)nitrilo, and N-(2-hydroxyethoxy)aminocarbonyl groups, respectively. It is a MEK1 and MEK2 inhibitor (IC50= 12 nM). Approved by the FDA for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation in combination with encorafenib. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of benzimidazoles, a member of bromobenzenes, a member of monofluorobenzenes, a hydroxamic acid ester and a secondary amino compound.
brand name
Mektovi
General Description
Class: dual threonine/tyrosine kinase; Treatment: melanoma with BRAF mutations; Other name: ARRY-162; Oral bioavailability = 50%; Elimination half-life = 3.5 h; Protein binding = 97%
Pharmacokinetics
In vitro, binimetinib inhibited extracellular signal-related kinase (ERK) phosphorylation in cellfree assays as well as viability and MEK-dependent phosphorylation of BRAF-mutant human melanoma cell lines. Binimetinib also inhibited in vivo ERK phosphorylation and tumor growth in BRAF-mutant murine xenograft models. MEK is an enzyme that regulates the biosynthesis of inflammatory cytokines such as TNF, IL-6 and IL-1; therefore, binimetinib anti-tumor activity can be mediated through the interference of cytokines biosynthesis.
Binimetinib and encorafenib target two different kinases in the RAS/RAF/MEK/ERK pathway. Compared to either drug alone, coadministration of encorafenib and binimetinib resulted in greater anti-proliferative activity in vitro in BRAF mutation-positive cell lines and greater anti-tumor activity with respect to tumor growth inhibition in BRAF V600E mutant human melanoma xenograft studies in mice. Additionally, the combination of binimetinib and encorafenib delayed the emergence of resistance in BRAF V600E mutant human melanoma xenografts in mice compared to either drug alone. In a BRAF V600E mutant NSCLC patient-derived xenograft model in mice, coadministration of encorafenib and binimetinib resulted in greater anti-tumor activity compared to binimetinib alone, with respect to tumor growth inhibition. Increased tumor growth delay after dosing cessation was also observed with the coadministration compared to either drug alone.
Following MEKTOVI 45 mg twice daily, no clinically meaningful QT prolongation was observed.
Pharmacokinetics
After oral administration, binimetinib is absorbed rapidly, with a median tmax of 1.48 h. Binimetinib is 50% orally bioavailable and exhibits a short elimination half-life of 3.5 h. Consequently, it requires twice-daily dosing regimen. Binimetinib undergoes UGT1A1-mediated glucuronidation, which contributes up to 61% of the overall metabolism. Other metabolic pathways include N-dealkylation, amide hydrolysis, and loss of ethanediol from the side chain.
Metabolism
The primary metabolic pathway is glucuronidation with UGT1A1 contributing up to 61% of the binimetinib metabolism. Other pathways of binimetinib metabolism include N-dealkylation, amide hydrolysis, and loss of ethane-diol from the side chain. The active metabolite M3 produced by CYP1A2 and CYP2C19 represents 8.6% of the binimetinib exposure. Following a single oral dose of 45 mg radiolabeled binimetinib, approximately 60% of the circulating radioactivity AUC in plasma was attributable to binimetinib.
References
1) Lee?et al.?(2010),?Preclinical development of ARRY-162, a potent and selective MEK1/2 inhibitor;?Cancer Res.?70?2515 2) Winski?et al.?(2010),?MEK162 (ARRY-162), a novel MEK ? inhibitor, inhibits tumor growth regardless of KRAS/RAF pathway mutations;?EJC Supplements?8?56 3) Lee?et al.?(2016),?Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models;?Oncotarget?7?39595 4) Gong?et al.?(2017),?MEK162 Enhances Antitumor Activity of 5-Fluorouracil and Trifluridine in KRAS-mutated Human Colorectal Cancer Cell Lines;?Anticancer Res.?37?2831 5) Van Cutsem?et al.?(2019),?Binimetinib, Encorafenib, and Cetuximab Triplet Therapy for Patients With BRAF V600E-Mutant Metastatic Colorectal Cancer: Safety Lead-In Results From Phase III BEACON Colorectal Cancer study;?J. Clin. Oncol.?180?2459
Properties of Binimetinib
| Melting point: | >203oC (dec.) |
| Density | 1.67 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to at least 25 mg/ml) |
| form | solid |
| pka | 14.20±0.10(Predicted) |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for Binimetinib
Computed Descriptors for Binimetinib
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Mek162 95% CAS 606143-89-9View Details
Mek162 95% CAS 606143-89-9View Details
606143-89-9 -
 MEK162 98% (HPLC) CAS 606143-89-9View Details
MEK162 98% (HPLC) CAS 606143-89-9View Details
606143-89-9 -
 Binimetinib CAS 606143-89-9View Details
Binimetinib CAS 606143-89-9View Details
606143-89-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1