L-Phenylalanine
Synonym(s):L-Phenylalanine;Phe;Phe, 2-Amino-3-phenylpropionic acid;L-Phenylalanine - CAS 63-91-2 - Calbiochem;(S)-2-Amino-3-phenylpropionic acid
- CAS NO.:63-91-2
- Empirical Formula: C9H11NO2
- Molecular Weight: 165.19
- MDL number: MFCD00064227
- EINECS: 200-568-1
- Update Date: 2025-12-17 09:49:33
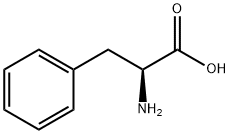
What is L-Phenylalanine?
Absorption
Absorbed from the small intestine by a sodium dependent active transport process.
Toxicity
L-phenylalanine will exacerbate symptoms of phenylketonuria if used by phenylketonurics. L-phenylalanine was reported to exacerbate tardive dyskinesia when used by some with schizophrenia.
Chemical properties
White crystalline powder
Chemical properties
L-Phenylalanine has no odor and a slight bitter taste. It melts with decomposition at about 283°C. The pH of a 1 in 100 solution is between 5.4 and 6.0. FEMA notes this chemical is used in cocoa substitute only.
Occurrence
Reported found in white bread, macaroni, egg noodles, corn flakes, corn grits, oatmeal, wheat bran, wheat flakes, shredded wheat, barley, brown rice, rye flour, whole grain wheat flour, buttermilk, blue cheese, cheddar cheese, cottage cheese, cream cheese, parmesan cheese, bacon, cured ham, frankfurters, pork sausage, canned red kidney beans, canned sweet corn, canned peas, canned lima beans, canned potatoes, canned asparagus, canned snap beans, canned beets, beef, lamb, fresh ham, veal round, beef liver, chicken, chicken liver, turkey and other natural sources.
The Uses of L-Phenylalanine
L-Phenylalanine is an essential amino acid. L-Phenylalanine is biologically converted into L-tyrosine, another one of the DNA-encoded amino acids, which in turn is converted to L-DOPA and further conv erted into dopamine, norepinephrine, and epinephrine. L-Phenylalanine is produced for medical, feed, and nutritional applications such as in the preparation of Aspartame.
The Uses of L-Phenylalanine
L-phenylalanine is an amino acid used as a skin-conditioning agent. It has greater use in hair care than in skin care products.
The Uses of L-Phenylalanine
phenylalanine is a conditioning agent with greater application in hair care than in skin care preparations. It is also used in suntan products.
Background
Phenylalanine is an essential aromatic amino acid that is a precursor of melanin, dopamine, noradrenalin (norepinephrine), and thyroxine.
Indications
L-phenylalanine may be helpful in some with depression. It may also be useful in the treatment of vitiligo. There is some evidence that L-phenylalanine may exacerbate tardive dyskinesia in some schizophrenic patients and in some who have used neuroleptic drugs.
Definition
ChEBI: The L-enantiomer of phenylalanine.
Preparation
From PTS-negative Escherichia coli bioengineered strains.
Production Methods
In previous large-scale production processes for L-phenylalanine two enzymatic methods were applied: 1. Resolution of N-acetyl-D,L-phenylalanine by carrier-fixed microbial acylase: This process provided pharmaceutical-grade L-phenylalanine, but suffered from the disadvantage that the D-enantiomer had to be racemized and recycled. 2. Stereoselective and enantioselective addition of ammonia to trans-cinnamic acid, catalyzed by L-phenylalanine ammonia lyase (PAL, EC 4.3.1.5): PAL-containing Rhodotorula rubra was used in an industrial process to supply L-phenylalanine for the first production campaign of the sweetener aspartame. When continuously operated in an immobilized whole cell reactor, the bioconversion reached concentration up to 50 g/L Lphenylalanine at a conversion of about 83%. Other processes started from phenylpyruvate with L-aspartic acid as amine donor using immobilized cells of Escherichia coli or from a-acetamidocinnamic acid and immobilized cells of a Corynebacterium equi strain. In both cases L-phenylalanine concentrations up to 30 g/L and more (molar yields as high as at least 98 %) were reached.
However, fermentation processes based on glucose-consuming L-phenylalanine overproducing ;mutants of E. coli and coryneform strains turned out to be more economical. L-Phenylalanine is formed in ten enzymatic steps starting from erythrose-4-phosphate and phosphoenolpyruvate. A suitable profile of the specific glucose feed rate prevents acetate formation and leads to improved L-phenylalanine production with a final concentration up to 46 g/L and a corresponding yield of 18 %.
L-Phenylalanine is recovered from the fermentation broth either by two-step crystallization or by an ionexchange resin process. The preferred cell separation technique is ultrafiltration; and the filtrates may be treated with activated carbon for further purification. Instead of ion-exchange resins nonpolar, highly porous synthetic adsorbents are recommended to remove impurities. An alternative process in which a cell separator is integrated in the fermentation part, thus allowing cell recycling, was suggested for L-phenylalanine production and may lead to prospective developments.
Synthesis Reference(s)
Canadian Journal of Chemistry, 29, p. 427, 1951 DOI: 10.1139/v51-051
The Journal of Organic Chemistry, 30, p. 3414, 1965 DOI: 10.1021/jo01021a035
Tetrahedron Letters, 26, p. 2449, 1985 DOI: 10.1016/S0040-4039(00)94850-0
General Description
Odorless white crystalline powder. Slightly bitter taste. pH (1% aqueous solution) 5.4 to 6.
Air & Water Reactions
Water soluble. Aqueous solutions are weak acids.
Reactivity Profile
L-Phenylalanine may be light sensitive. Act as weak acids in solution.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition L-Phenylalanine emits toxic fumes of nitrogen oxides.
Fire Hazard
Flash point data for L-Phenylalanine are not available; however, L-Phenylalanine is probably combustible.
Biochem/physiol Actions
L-Phenylalanine is an essential amino acid. It is significantly involved in the synthesis of neurotransmitters such as dopamine, epinephrine, norepinephrine, l-DOPA (Dihydroxyphenylalanine), melanin and thyroxine. L-Phenylalanine metabolism also results in phenylethylamine, that brings about effect of a stimulant in the brain and enhances mood.
Pharmacokinetics
Used by the brain to produce Norepinephrine, a chemical that transmits signals between nerve cells and the brain; keeps you awake and alert; reduces hunger pains; functions as an antidepressant and helps improve memory.
Safety Profile
Mildly toxic by intraperitoneal route. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Hepatic. L-phenylalanine that is not metabolized in the liver is distributed via the systemic circulation to the various tissues of the body, where it undergoes metabolic reactions similar to those that take place in the liver.
Purification Methods
Likely impurities are leucine, valine, methionine and tyrosine. Crystallise L-phenylalanine from water by adding 4volumes of EtOH. Dry it in vacuo over P2O5. Also crystallise it from saturated refluxing aqueous solutions at neutral pH, or 1:1 (v/v) EtOH/water solution, or conc HCl. It sublimes at 176-184o/0.3mm with 98.7% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2156-2175 1961, Beilstein 14 IV 1552.]
Properties of L-Phenylalanine
| Melting point: | 270-275 °C (dec.)(lit.) |
| Boiling point: | 293.03°C (rough estimate) |
| alpha | -34.1 º (c=2, water, dry basis) |
| Density | 1.29 |
| vapor pressure | <1 Pa (25 °C) |
| FEMA | 3585 | L-PHENYLALANINE |
| refractive index | -34 ° (C=2, H2O) |
| storage temp. | Store below +30°C. |
| solubility | H2O: 0.1 M at 20 °C, clear, colorless |
| form | powder |
| pka | 2.2(at 25℃) |
| color | White to off-white |
| PH | 5.0-7.0 (25℃, 0.1M in H2O) |
| Odor | at 100.00 %. odorless |
| optical activity | [α]25/D -34.2°, c = 2 in H2O (dried basis) |
| Water Solubility | 1-5 g/100 mL at 25 ºC |
| Merck | 14,7271 |
| JECFA Number | 1428 |
| BRN | 1910408 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 63-91-2(CAS DataBase Reference) |
| NIST Chemistry Reference | L-Phenylalanine(63-91-2) |
| EPA Substance Registry System | L-Phenylalanine (63-91-2) |
Safety information for L-Phenylalanine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for L-Phenylalanine
| InChIKey | COLNVLDHVKWLRT-QMMMGPOBSA-N |
L-Phenylalanine manufacturer
JSK Chemicals
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

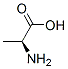

You may like
-
 L-Phenylalanine 99%View Details
L-Phenylalanine 99%View Details -
 L-Phenylalanine extrapure CHR CAS 63-91-2View Details
L-Phenylalanine extrapure CHR CAS 63-91-2View Details
63-91-2 -
 L-Phenylalanine CAS 63-91-2View Details
L-Phenylalanine CAS 63-91-2View Details
63-91-2 -
 L-Phenyl alanine CAS 63-91-2View Details
L-Phenyl alanine CAS 63-91-2View Details
63-91-2 -
 L-Phenylalanine for cell culture CAS 63-91-2View Details
L-Phenylalanine for cell culture CAS 63-91-2View Details
63-91-2 -
 L-Phenylalanine ExiPlus CAS 63-91-2View Details
L-Phenylalanine ExiPlus CAS 63-91-2View Details
63-91-2 -
 L-Phenylalanine (H-Phe-OH) 99% CAS 63-91-2View Details
L-Phenylalanine (H-Phe-OH) 99% CAS 63-91-2View Details
63-91-2 -
 L-Phenylalanine, CAS:63-91-2View Details
L-Phenylalanine, CAS:63-91-2View Details
63-91-2
