L-Threonine
Synonym(s):(2S,3R)-2-Amino-3-hydroxybutyric acid;L-Threonine;Thr, 2-Amino-3-hydroxybutanoic acid
- CAS NO.:72-19-5
- Empirical Formula: C4H9NO3
- Molecular Weight: 119.12
- MDL number: MFCD00064270
- EINECS: 200-774-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
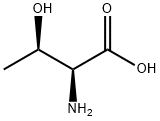
What is L-Threonine?
Description
L-Threonine is an essential amino acid, that is, humans must obtain it from foods because their bodies cannot synthesize it. Foods that contain high amounts of protein, such as meats, fish, eggs, and dairy products, are good sources of L-threonine.
L-Threonine, abbreviated Thr, is the last common amino acid to be discovered. Although biochemist William C. Rose and colleagues at the University of Illinois (Urbana–Champaign) are usually credited with discovering Thr in 1936, biochemist Samuel B. Schryver and botanist H. W. Buston at Imperial College London reported isolating it from oat protein in 1926.
Threonine has two chiral centers and therefore four possible stereoisomers. The isomers other than Thr (D-, L-allo-, and D-allo-threonine) are rare in nature and have no value, nutritional or otherwise.
These days, most Thr is synthesized rather than being obtained from natural proteins. But this past April, Evonik Industries (Essen, Germany) feeling competitive pressure, closed its L-threonine plant in Kaba, Hungary, causing ≈120 people to lose their jobs.
Chemical properties
A white or almost white, crystalline powder or colourless crystals.
The Uses of L-Threonine
L-Threonine is used to alleviate anxiety and mild depression. It is an important component of many proteins such as tooth enamel, collagen, and elastin. It is an important amino acid in the nervous system and plays a vital role in porphyrin and fat metabolism. It is useful in intestinal disorders and indigestion. It is also used as a prodrug to reliably elevate brain glycine levels.
The Uses of L-Threonine
amino acid, nutrient
The Uses of L-Threonine
L-enantiomer
Background
An essential amino acid occurring naturally in the L-form, which is the active form. It is found in eggs, milk, gelatin, and other proteins.
Indications
L-Threonine makes up collagen, elastin, and enamel protein. It aids proper fat metabolism in the liver, helps the digestive and intestinal tracts function more smoothly, and assists in metabolism and assimilation.
Definition
ChEBI: An optically active form of threonine having L-configuration.
brand name
L -Threonine is JAN.
Biotechnological Production
L-Threonine can be produced using strains of E. coli or C. glutamicum. As threonine is also an amino acid of the aspartate family, aspartate semialdehyde is a common intermediate with the biosynthesis of L-lysine. In order to optimize a high-yielding L-threonine–producing strain, the following strategy is applied: the pathway towards L-lysine is minimized by reducing the activity of dihydrodipicolinate synthase (dapA) and at the same time the pathway towards L-threonine is favored by overexpression of the genes of the threonine operon, which consists of the genes for homoserine dehydratase (thrA), homoserine kinase (thrB), and threonine synthase (thrC). As L-threonine is also a precursor for L-isoleucine, further conversion of L-threonine into L-isoleucine has to be minimized by deactivation of the threonine dehydratase gene (ilvA). In the meantime, E. coli based strains have also been developed by the application of systems biology, not only by deletion or downregulation of the competing pathways such as L-lysine, L-methionine, and L-isoleucine, but also by optimization of the supply of key precursors such as oxaloacetate. The E. coli strain has been reported to produce 82 g/L L-threonine in 48 h with a carbon yield of 39 %. A more detailed description of the development of a commercial L-threonine process has been given by Debabov. Today L-threonine is manufactured on a commercial scale of several thousand tonnes using the E. coli fermentation process.
Biochem/physiol Actions
L-Threonine is considered to be an essential amino acid. L-Threonine is involved in the synthesis of mucin, a protein supporting intestinal function and integrity. L-Threonine is required for the performance of the immune system. It is also essential for O-linked glycosylation, protein phosphorylation and glycine synthesis.
Pharmacokinetics
L-Threonine is an essential amino acid that helps to maintain the proper protein balance in the body. It is important for the formation of collagen, elastin, and tooth enamel, and aids liver and lipotropic function when combined with aspartic acid and methionine.
Safety Profile
Moderately toxic by intraperitoneal route. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Hepatic
Purification Methods
Likely impurities are allo-threonine and glycine. Crystallise L-threonine from H2O by adding 4volumes of EtOH. Dry and store it in a desiccator. It also crystallises from 80% EtOH to give hexagonal plates m 262-263o(dec). It sublimes at 200-226o/0.3mm with 99.6% recovery and unracemised [Gross & Gradsky J Am Chem Soc 77 1678 1955]. [Elliot J Chem Soc 62 1950, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 1 pp 176-183, Vol 3 pp 2238-2257 1961, Beilstein 4 IV 3171.]
Properties of L-Threonine
| Melting point: | 256 °C (dec.) (lit.) |
| Boiling point: | 222.38°C (rough estimate) |
| alpha | -28.4 º (c=6, H2O) |
| Density | 1.3126 (rough estimate) |
| refractive index | -28 ° (C=6, H2O) |
| FEMA | 4710 | L-THREONINE |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| appearance | colorless crystals |
| pka | 2.09(at 25℃) |
| color | Yellow |
| PH | 5-6 (100g/l, H2O, 20℃) |
| Odor | ADM L-Threonine is a high quality product specifically designed for the feed industry. Produced from advanced technology, ADM L-Threonine is composed of 100% isomerically pure L-Threonine, which translates into 100% bioavailability for swine, poultry, and other animals.mild savory |
| optical activity | [α]20/D 28.5±0.5°, c = 5% in H2O |
| Water Solubility | 90 g/L (20 ºC) |
| Merck | 14,9380 |
| JECFA Number | 2119 |
| BRN | 1721646 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 72-19-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Threonine(72-19-5) |
| EPA Substance Registry System | L-Threonine (72-19-5) |
Safety information for L-Threonine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for L-Threonine
| InChIKey | AYFVYJQAPQTCCC-GBXIJSLDSA-N |
L-Threonine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 L-Threonine CAS 72-19-5View Details
L-Threonine CAS 72-19-5View Details
72-19-5 -
 L-(-)-Threonine CAS 72-19-5View Details
L-(-)-Threonine CAS 72-19-5View Details
72-19-5 -
 L-Threonine, for biochemistry CAS 72-19-5View Details
L-Threonine, for biochemistry CAS 72-19-5View Details
72-19-5 -
 L-Threonine for cell culture CAS 72-19-5View Details
L-Threonine for cell culture CAS 72-19-5View Details
72-19-5 -
 L-Threonine extrapure CHR CAS 72-19-5View Details
L-Threonine extrapure CHR CAS 72-19-5View Details
72-19-5 -
 L-Threonine 99% CAS 72-19-5View Details
L-Threonine 99% CAS 72-19-5View Details
72-19-5 -
 L ThreonineView Details
L ThreonineView Details
56-45-1 -
 L-ThreonineView Details
L-ThreonineView Details
72-19-5
