L-Hyoscyamine
Synonym(s):;LP;SEPSECS;SLA;SLA/LP
- CAS NO.:101-31-5
- Empirical Formula: C17H23NO3
- Molecular Weight: 289.37
- MDL number: MFCD00067306
- EINECS: 202-933-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
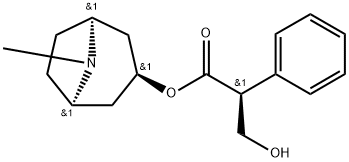
What is L-Hyoscyamine?
Absorption
Hyoscyamine is completely absorbed by sublingual and oral routes, though exact data regarding the Cmax, Tmax, and AUC are not readily available.
Toxicity
Patients experiencing an overdose may present with headache, nausea, vomiting, dizziness, dry mouth, difficulty in swallowing, dilated pupils, blurred vision, urinary retention, hot dry and flushed skin, tachycardia, hypertension, hypotension, respiratory depression, CNS stimulation, fever, ataxia, excitation, lethargy, stupor, coma, and paralysis. Patients should be treated with symptomatic and supportive therapy which may include emesis, gastric lavage, activated charcoal, artificial respiration, or intravenous physostigmine. Dialysis is expected to remove hyoscyamine sulfate from circulation.
Chemical properties
White to Off-White Solid
The Uses of L-Hyoscyamine
L-Hyoscyamine can be used as anticholinergic and analgesic.
The Uses of L-Hyoscyamine
L-Hyoscyamine is a natural compound that has inhibitory activity against cholinesterases.
The Uses of L-Hyoscyamine
Suggested starting dilutions are as follows: IHC-P: 1:100-1:1000, WB: 1:1000-1:10000. Not yet tested in other applications. Optimal working dilutions should be determined experimentally by the end user.
Indications
As a drug that is not FDA approved, hyscyamine has no official indications. Intravenous hysocyamine has been used to reduce gastric motility, reduce pancreatic pain and secretions, to facilitate imaging of the gastrointestinal tract, treat anticholinesterase toxicity, treat certain cases of partial heart block, improve visualization of the kidneys, and for symptomatic relief of biliary and renal colic. Intravenous hyoscyamine is also used pre-operatively to reduce secretions of the mouth and respiratory tract to facilitate intubation. Oral hyoscyamine is used to treat functional intestinal disorders, for symptomatic relief of biliary and renal colic, and symptomatic relief of acute rhinitis.
Background
Hyoscyamine is a tropane alkaloid and the levo-isomer of atropine. It is commonly extracted from plants in the Solanaceae or nightshade family. Research into the action of hyoscyamine in published literature dates back to 1826. Hyoscyamine is used for a wide variety of treatments and therapeutics due to its antimuscarinic properties.
Although hyoscyamine is marketed in the United States, it is not FDA approved.
What are the applications of Application
L-Hyoscyamine is a non-selective, competitive antagonist of the muscarinic acetylcholine receptors
Definition
ChEBI: An atropine with a 2S-configuration.
General Description
Hyoscyamine is a levorotatory alkaloidobtained from various solanaceous species. One ofthe commercial sources is Egyptian henbane (Hyoscyamusmuticus), in which it occurs to the extent of about 0.5%.Usually, it is prepared from the crude drug in a manner similarto that used for atropine and is purified as the oxalate.The free base is obtained easily from this salt.
It occurs as white needles that are sparingly soluble inwater (1:281), more soluble in ether (1:69) or benzene(1:150), very soluble in chloroform (1:1), and freely solublein alcohol. It is used as the sulfate and hydrobromide. Theprincipal reason for the popularity of the hydrobromide hasbeen its nondeliquescent nature. The salts have the advantageover the free base in being quite water soluble.
Biochem/physiol Actions
The 21st amino acid, selenocysteine (sec), is distinct from other amino acids because it lacks its own tRNA synthetase and is the only amino acid synthesized on its cognate tRNA. Synthesis of sec begins with acylation of tRNA(sec) (TRSP; MIM 165060) by seryl-tRNA synthetase (SARS; MIM 607529) to give ser-tRNA(sec), which is subsequently phosphorylated by O-phosphoseryl-tRNA kinase (PSTK; MIM 611310) to give O-phosphoseryl-tRNA(sec). SEPSECS catalyzes the final step of sec synthesis by converting O-phosphoseryl-tRNA(sec) to selenocysteinyl-tRNA(sec) using selenophosphate as the selenium donor (Palioura et al., 2009 [PubMed 19608919]).[supplied by OMIM]
Pharmacokinetics
Hyoscyamine is not FDA approved, and so it has not official indications. However, it is used as an antimuscarinic agent in a number of treatments and therapies. Hyoscyamine has a short duration of action as it may need to be given multiple times per day. Patients should be counselled regarding the risks and signs of anticholinergic toxicity.
Clinical Use
(L-Hyoscyamine)Hyoscyamine is used to treat disorders of the urinarytract more so than any other antispasmodic, although thereis no evidence that it has any advantages over the otherbelladonna preparations and the synthetic anticholinergics.It is used to treat spasms of the bladder and, in this manner,serves as a urinary stimulant. It is used together witha narcotic to counteract the spasm produced by the narcoticwhen the latter is used to relieve the pain of urethral colic.Hyoscyamine preparations are also used as antispasmodicsin the therapy of peptic ulcers.
Metabolism
Hyoscyamine is largely unmetabolized, however a small amount is hydrolyzed into tropine and tropic acid.
Properties of L-Hyoscyamine
| Melting point: | 108,5°C |
| Boiling point: | 431.53°C (rough estimate) |
| alpha | D20 -21.0° (alc) |
| Density | 1.0470 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO:79.0(Max Conc. mg/mL);273.0(Max Conc. mM) Ethanol:58.0(Max Conc. mg/mL);200.43(Max Conc. mM) |
| pka | pKa (21°) 9.7 |
| form | powder |
| color | white |
| Water Solubility | 3.6g/L(20 ºC) |
| Merck | 14,4858 |
| CAS DataBase Reference | 101-31-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Hyoscyamine(101-31-5) |
| EPA Substance Registry System | Benzeneacetic acid, .alpha.-(hydroxymethyl)-, (3-endo)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester, (.alpha.S)- (101-31-5) |
Safety information for L-Hyoscyamine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P284:Wear respiratory protection. P307+P311:IF exposed: call a POISON CENTER or doctor/physician. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for L-Hyoscyamine
| InChIKey | RKUNBYITZUJHSG-FXUDXRNXSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
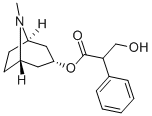



You may like
-
 101-31-5 (-)-Hycosamine 98%View Details
101-31-5 (-)-Hycosamine 98%View Details
101-31-5 -
 101-31-5 98%View Details
101-31-5 98%View Details
101-31-5 -
 Anti-SEPSECS antibody produced in rabbit CASView Details
Anti-SEPSECS antibody produced in rabbit CASView Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
