L-Aspartic acid
Synonym(s):(S)-(+)-Aminosuccinic acid;(S)-Aminobutanedioic acid
- CAS NO.:56-84-8
- Empirical Formula: C4H7NO4
- Molecular Weight: 133.1
- MDL number: MFCD00002616
- EINECS: 200-291-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
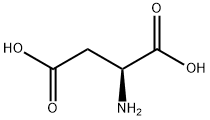
What is L-Aspartic acid ?
Absorption
Absorbed from the small intestine by an active transport process
Toxicity
Mild gastrointestinal side effects including diarrhea. LD50 (rat) > 5,000 mg/kg.
Description
L-Aspartic acid is the L-form of the aspartic acid. It is one of the 20 amino acids that used in the protein synthesis. It is the non-essential amino acids for humans as it can be synthesized in vivo. It is important in the synthesis of other amino acids and some nucleotides, and is a metabolite in the citric acid and urea cycles. In animals, it may be used as a neurotransmitter. It can be chemically synthesize from the diethyl sodium phthalimidomalonate. Currently, almost all the aspartic acids are manufactured in China. Its application include being used as low calorie sweetener (as the part of the aspartame), scale and corrosion inhibitor, and in resins. One of its growing applications is for the manufacturing of biodegradable superabsorbent polymer, polyaspartic acid. It can also be used in fertilizer industry to improve water retention and nitrogen uptake.
Chemical properties
Colorless crystals. Soluble in water; insoluble in alcohol and ether. Optically active. dl-aspartic acid.
Chemical properties
Aspartic acid (abbreviated as Asp or D) is an α-amino acid with the chemical formula HOOCCH(NH2 )CH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The Lisomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins. Its codons are GAU and GAC. Aspartic acid is, together with glutamic acid, classified as an acidic amino acid with a pKa of 3.9, however in a peptide the pKa is highly dependent on the local environment. A pKa as high as 14 is not at all uncommon. Aspartate is pervasive in biosynthesis. As with all amino acids, the presence of acid protons depends on the residue's local chemical environment and the pH of the solution.
Chemical properties
Apartic acid is an aliphatic monoaminodicarboxylic acid (amino acid) and is a well-known constituent of protein. It has a slight acid taste
Physical properties
Solubility 0.5 (25 ℃) g/100 g H2O, pI 2.98, dissociation constants: pK1 2.1, pK2 3.86 (β-COOH), pK3 9.82
Occurrence
Dietary sources
Aspartic acid is not an essential amino acid, which means that it can be synthesized from central metabolic pathway intermediates in humans. Aspartic acid is found in :
Animal sources : luncheon meats, sausage meat, wild game
Vegetable sources: sprouting seeds, oat flakes, avocado,
asparagus , young sugarcane, and molasses from sugar beets.
Chemical synthesis
Racemic aspartic acid can be synthesized from diethyl sodium phthalimido malonate, (C6H4(CO)2NC(CO2Et)2).
The major disadvantage of the above technique is that equimolar amounts of each enantiomer are made. Using biotechnology it is now possible to use immobilized enzymes to create just one type of enantiomer owing to their stereo specificity. Aspartic acid is made synthetically using ammonium fumarate and aspartase from E.coli, E.coli usually breaks down the aspartic acid as a nitrogen source but using excess amounts of ammonium fumarate a reversal of the enzyme's job is possible, and so aspartic acid is made to very high yields, 98.7 mM from 1 M.
History
Aspartic acid was first discovered in 1827 by Plisson, derived from asparagine, which had been isolated from asparagus juice in 1806, by boiling with a base.
The Uses of L-Aspartic acid
L-aspartic acid is used as a dietary supplement, it can be blended with minerals to make compounds like potassium aspartate, copper aspartate, manganese aspartate, magnesium aspartate, zinc aspartate and more. Increasing the absorption, and hence utilization potentials, of these minerals via the addition of aspartate induces certain health benefits. Many athletes use L-aspartic acid-based mineral supplements orally to enhance their performance capacities. Aspartic acid and glutamic acid play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. It can help promote a robust metabolism, and is sometimes used to treat fatigue and depression. Aspartic acid is used as a component of parenteral and enteral nutrition. In pharmaceutical agents aspartic acid is used as an ammoniac detoxicating agent, hepar function accelerator and fatigue refresher.
The Uses of L-Aspartic acid
l-aspartic acid is an amino acid used as a skin-conditioning agent.
The Uses of L-Aspartic acid
L-Aspartic acid is used as a component of parenteral and enteral nutrition and as a pharmaceutical ingredient. it is used for cell culture and in manufacturing processes. It is widely utilized for mineral supplementation in the salt form.
Background
One of the non-essential amino acids commonly occurring in the L-form. It is found in animals and plants, especially in sugar cane and sugar beets. It may be a neurotransmitter.
Indications
There is no support for the claim that aspartates are exercise performance enhancers, i.e. ergogenic aids.
Definition
ChEBI: The L-enantiomer of aspartic acid.
Aroma threshold values
Detection: 300 ppb
General Description
To request documentation for this product, please contact Customer Support and select ‘Product Documentation′. Please note that access to the documentation for this product requires a confidentiality disclosure agreement.
Hazard
Low toxicity.
Biological Activity
Endogenous NMDA receptor agonist.
Biochem/physiol Actions
Principal neurotransmitter for fast synaptic excitation.
Pharmacokinetics
L-aspartate is considered a non-essential amino acid, meaning that, under normal physiological conditions, sufficient amounts of the amino acid are synthesized in the body to meet the body's requirements. L-aspartate is formed by the transamination of the Krebs cycle intermediate oxaloacetate. The amino acid serves as a precursor for synthesis of proteins, oligopeptides, purines, pyrimidines, nucleic acids and L-arginine. L-aspartate is a glycogenic amino acid, and it can also promote energy production via its metabolism in the Krebs cycle. These latter activities were the rationale for the claim that supplemental aspartate has an anti-fatigue effect on skeletal muscle, a claim that was never confirmed.
Safety Profile
Low toxicity by intraperitoneal route. When heated to decomposition emits toxic fumes of NOx.
Synthesis
Aspartate is non - essential in mammals, being produced from oxaloacetate by transamination. It can also be generated from ornithine and citrulline in the urea cycle. In plants and microorganisms, aspartate is the precursor to several amino acids, including four that are essential for humans: methionine, threonine, isoleucine, and lysine. The conversion of aspartate to these other amino acids begins with reduction of aspartate to its "semi aldehyde," O2CCH(NH2)CH2CHO. Asparagine is derived from aspartate via trans amidation :
-O2CCH(NH2)CH2CO2 - + G C (O)NH3+ O2CCH(NH2)CH2CONH3+ + GC(O)O
(where GC(O)NH2 and GC(O)OH are glutamine and glutamic acid, respectively).
Synthesis
Enzymatically, aspartic acid is reversibly synthesized by a transamination reaction between oxaloacetic acid and glutamic acid in the presence of pyridoxal phosphate.
Metabolism
Not Available
storage
Room temperature
Forms and nomenclature
There are two forms or enantiomers of aspartic acid. The name "aspartic acid" can refer to either enantiomer or a mixture of two. Of these two forms, only one, "L - aspartic acid", is directly incorporated into proteins. The biological roles of its counterpart, "Daspartic acid" are more limited. Where enzymatic synthesis will produce one or the other, most chemical syntheses will produce both forms, "DL-aspartic acid," known as a racemic mixture.
Other biochemical roles
Aspartate is also a metabolite in the urea cycle and participates in gluconeogenesis. It carries reducing equivalents in the malateaspartate shuttle, which utilizes the ready inter conversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartate donates one nitrogen atom in the biosynthesis of inosine, the precursor to the purine bases. In addition, aspartic acid acts as hydrogen acceptor in a chain of ATP synthase.
Properties of L-Aspartic acid
| Melting point: | >300 °C (dec.)(lit.) |
| Boiling point: | 245.59°C (rough estimate) |
| alpha | 25 º (c=8, 6N HCl) |
| Density | 1.66 |
| refractive index | 1.4540 (estimate) |
| FEMA | 3656 | L-ASPARTIC ACID |
| storage temp. | Store below +30°C. |
| solubility | H2O: 5 mg/mL |
| form | powder |
| pka | 1.99(at 25℃) |
| color | White |
| Odor | acid taste |
| PH | 2.5-3.5 (4g/l, H2O, 20℃) |
| optical activity | [α]20/D +24.7±1°, c = 5% in 5 M HCl |
| Water Solubility | 5 g/L (25 ºC) |
| λmax | λ: 260 nm Amax: 0.20 λ: 280 nm Amax: 0.10 |
| Merck | 14,840 |
| JECFA Number | 1429 |
| BRN | 1723530 |
| Stability: | Stable. Combustible. Incompatible with strong oxidising agents. |
| CAS DataBase Reference | 56-84-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Aspartic acid(56-84-8) |
| EPA Substance Registry System | Aspartic acid (56-84-8) |
Safety information for L-Aspartic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for L-Aspartic acid
| InChIKey | CKLJMWTZIZZHCS-REOHCLBHSA-N |
L-Aspartic acid manufacturer
Aditya Chemicals
ARRAKIS INDUSTRIES LLP
SS Reagents and Chemicals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 56-84-8 L-Aspartic acid, 99% 98%View Details
56-84-8 L-Aspartic acid, 99% 98%View Details
56-84-8 -
 L-Aspartic acid, Low metals content CAS 56-84-8View Details
L-Aspartic acid, Low metals content CAS 56-84-8View Details
56-84-8 -
 L-Aspartic acid, Low metals content CAS 56-84-8View Details
L-Aspartic acid, Low metals content CAS 56-84-8View Details
56-84-8 -
 L-Aspartic Acid ExiPlus CAS 56-84-8View Details
L-Aspartic Acid ExiPlus CAS 56-84-8View Details
56-84-8 -
 L-Aspartic Acid extrapure CHR CAS 56-84-8View Details
L-Aspartic Acid extrapure CHR CAS 56-84-8View Details
56-84-8 -
 L-Aspartic acid CAS 56-84-8View Details
L-Aspartic acid CAS 56-84-8View Details
56-84-8 -
 L-Aspartic acid 99% CAS 56-84-8View Details
L-Aspartic acid 99% CAS 56-84-8View Details
56-84-8 -
 L-Aspartic acid CAS 56-84-8View Details
L-Aspartic acid CAS 56-84-8View Details
56-84-8