L-Malic acid
Synonym(s):(S)-(−)-2-Hydroxysuccinic acid;(S)-(-)-Malic acid;L -Hydroxybutanedioic acid;L-(-)-Malic acid
- CAS NO.:97-67-6
- Empirical Formula: C4H6O5
- Molecular Weight: 134.09
- MDL number: MFCD00064213
- EINECS: 202-601-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-21 21:32:25
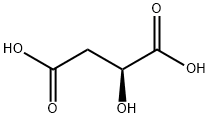
What is L-Malic acid?
Description
L-Malic acid is nearly odorless (sometimes a faint, acrid odor) with a tart, acidic taste. It is nonpungent. May be prepared by hydration of maleic acid; by fermentation from sugars.
Description
Malic acid, a hydroxydicarboxylic acid, is found in all forms of life. It exists naturally only as the L-enantiomer. It should not be confused with the similar sounding maleic? and malonic?acids.
L-Malic acid gives many fruits, particularly apples, their characteristic flavor. It is often referred to as “apple acid”. The word malic is derived from the Latin mālum, for which Malus, the genus that contains all apple species, is also named.
The global market size for malic acid (natural and manufactured1) is ≈US$200 million; the US market is ≈$35 million. The primary end use in the United States is for flavoring beverages, foods, and confectionaries, with much smaller quantities used in cosmetics and personal care products. The price of malic acid ranges from US$0.90 to $10.00/kg, depending on the purity, quantity, and end use.
1. The manufactured product is racemic.
Chemical properties
clear colourless solution
Chemical properties
L-Malic acid is nearly odorless (sometimes a faint, acrid odor). This compound has a tart, acidic, nonpungent taste.
Occurrence
Occurs in maple sap, apple, melon, papaya, beer, grape wine, cocoa, sake, kiwifruit and chicory root.
The Uses of L-Malic acid
L-Malic acid is used as a food additive, Selective α-amino protecting reagent for amino acid derivatives. Versatile synthon for the preparation of chiral compounds including κ-opioid receptor agonists, 1α,25-dihydroxyvitamin D3 analogue, and phoslactomycin B.
The Uses of L-Malic acid
The naturally occuring isomer is the L-form which has been found in apples and many other fruits and plants. Selective α-amino protecting reagent for amino acid derivatives. Versatile synthon for the preparation of chiral compounds including κ-opioid rece
The Uses of L-Malic acid
Intermediate in chemical synthesis. Chelating and buffering agent. Flavoring agent, flavor enhancer and acidulant in foods.
Definition
ChEBI: An optically active form of malic acid having (S)-configuration.
Preparation
L-Malic acid can be prepared by hydration of maleic acid; by fermentation from sugar.
General Description
L-Malic acid is an organic acid that is commonly found in wine. It plays an important role in wine microbiological stability.
Biochem/physiol Actions
L-Malic acid is a part of cellular metabolism. Its application is recognized in pharmaceutics. It is useful in the treatment of hepatic malfunctioning, effective against hyper-ammonemia. It is used as a part of amino acid infusion. L-Malic acid also serves as a nanomedicine in the treatment of brain neurological disorders. A TCA (Krebs cycle) intermediate and partner in the malic acid aspartate shuttle.
Purification Methods
Crystallise S-malic acid (charcoal) from ethyl acetate/pet ether (b 55-56o), keeping the temperature below 65o. Or dissolve it by refluxing in fifteen parts of anhydrous diethyl ether, decant, concentrate to one-third volume and crystallise it at 0o, repeatedly to constant melting point. [Beilstein 3 IV 1123.]
Properties of L-Malic acid
| Melting point: | 101-103 °C (lit.) |
| Boiling point: | 167.16°C (rough estimate) |
| alpha | -2 º (c=8.5, H2O) |
| Density | 1.60 |
| vapor pressure | 0Pa at 25℃ |
| FEMA | 2655 | L-MALIC ACID |
| refractive index | -6.5 ° (C=10, Acetone) |
| Flash point: | 220 °C |
| storage temp. | Store below +30°C. |
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| form | Powder |
| appearance | white crystalline powder, granules, or needles |
| color | White |
| Specific Gravity | 1.595 (20/4℃) |
| Odor | odorless |
| PH | 2.2 (10g/l, H2O, 20℃) |
| pka | (1) 3.46, (2) 5.10(at 25℃) |
| optical activity | [α]20/D 30±2°, c = 5.5% in pyridine |
| Water Solubility | soluble |
| Merck | 14,5707 |
| JECFA Number | 619 |
| BRN | 1723541 |
| CAS DataBase Reference | 97-67-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Butanedioic acid, hydroxy-, (s)-(97-67-6) |
| EPA Substance Registry System | Butanedioic acid, 2-hydroxy-, (2S)- (97-67-6) |
Safety information for L-Malic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for L-Malic acid
| InChIKey | BJEPYKJPYRNKOW-REOHCLBHSA-N |
L-Malic acid manufacturer
JSK Chemicals
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 97-67-6 99%View Details
97-67-6 99%View Details
97-67-6 -
 L-Malic acid, 99% CAS 97-67-6View Details
L-Malic acid, 99% CAS 97-67-6View Details
97-67-6 -
 L(-)-Malic acid 98% (HPLC) CAS 97-67-6View Details
L(-)-Malic acid 98% (HPLC) CAS 97-67-6View Details
97-67-6 -
 L-(-)-Malic acid CAS 97-67-6View Details
L-(-)-Malic acid CAS 97-67-6View Details
97-67-6 -
 L-(-)-Malic Acid CAS 97-67-6View Details
L-(-)-Malic Acid CAS 97-67-6View Details
97-67-6 -
 L-(−)-Malic acid CAS 97-67-6View Details
L-(−)-Malic acid CAS 97-67-6View Details
97-67-6 -
 L-(−)-Malic acid CAS 97-67-6View Details
L-(−)-Malic acid CAS 97-67-6View Details
97-67-6 -
 L-(−)-Malic acid CAS 97-67-6View Details
L-(−)-Malic acid CAS 97-67-6View Details
97-67-6
