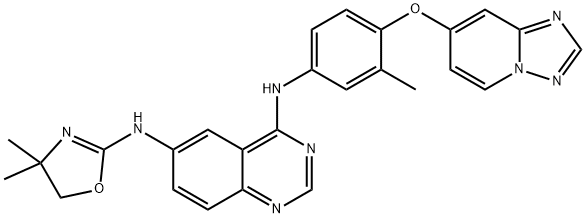
Tucatinib
- CAS NO.:937263-43-9
- Empirical Formula: C26H24N8O2
- Molecular Weight: 480.52
- MDL number: MFCD22380467
- EINECS: 200-001-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Tucatinib?
Absorption
The Tmax for tucatinib ranges from 1 to 4 hours. One pharmacokinetic study revealed a Cmax of 1120 ng/mL after a dose of 350 mg twice daily with a Tmax ranging from 1 to 3 hours. The AUCtau was reported to be about 7120 hours×ng/mL.
Toxicity
LD50 information and overdose information for tucatinib are not readily available in the literature. In the case of an overdose with this drug, increased adverse effects, such as diarrhea, nausea, abdominal pain, vomiting fatigue, hepatotoxicity, vomiting, decreased appetite, anemia, headache, and rash are expected.
Description
Irbinitinib is also known as ARRY-380 or Tucatinib. ARRY-380 is an orally inhibitor of human epidermal growth factor receptor tyrosine kinase ErbB-2 (also called HER2) with potential antineoplastic activity. It selectively binds to and inhibits the phosphorylation of ErbB-2, which may prevent the activation of ErbB-2 signal transduction pathways, resulting in growth inhibition and death of ErbB-2-expressing tumor cells. ErbB-2 is overexpressed in a variety of cancers and plays an important role in cellular proliferation and differentiation. Therefore, ARRY-380 may be an alternative treatmentmethod for the treatment of HER2+ cancers.
Characteristics
Year of discovery: 2006
Year of introduction: 2020
Discovered by: Array BioPharma
Developed by: Array BioPharma
Primary targets: HER2
Binding type: I/II
Class: receptor tyrosine kinase
Treatment: HER2-positive breast cancer
Other names: ONT-380, ARRY-380
Oral bioavailability = not reported
Elimination half-life = 5.4 hours
Protein binding = 97%
The Uses of Tucatinib
N6-(4,5-Dihydro-4,4-dimethyl-2-oxazolyl)-N4-[3-methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-4,6-quinazolinediamine is a novel?diarylamine ErbB inhibitor.
Background
Tucatinib is a kinase inhibitor drug used with trastuzumab and capecitabine in the treatment of unresectable or metastatic HER-2 positive breast cancer. It was developed by Seattle Genetics and approved by the FDA on April 17, 2020. Tucatinib is a promising new treatment for patients with metastatic breast cancer who have not responded adequately to other chemotherapy regimens.
Indications
Tucatinib is indicated with trastuzumab and capecitabine for the treatment of adults diagnosed with advanced unresectable or metastatic HER2-positive breast cancer. This includes patients with brain metastases and those who have received one or more prior anti-HER2-based regimens in the metastatic setting.
It is also indicated in combination with trastuzumab for the treatment of adult patients with RAS wild-type HER2-positive unresectable or metastatic colorectal cancer that has progressed following treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy. This indication is approved under accelerated approval; thus, it is contingent upon verification and description of clinical benefit in confirmatory trials.
brand name
TukysaTM
Pharmacokinetics
By inhibiting tyrosine kinase, tucatinib exerts anti-tumor activity, reducing the size of HER-2 positive breast cancer tumors. In clinical trials, the regimen of tucatinib and trastuzumab showed enhanced activity both in vitro and in vivo when compared to either drug administered by itself.
Metabolism
Tucatinib is metabolized by CYP2C8 with some contributions from CYP3A.
storage
Store at -20°C
Mode of action
Tucatinib binds to tyrosine kinase (an enzyme) of HER2, reducing PI3-kinase and MAP-kinase signaling. In vitro, tucatinib inhibits phosphorylation of HER2 and HER3, resulting in inhibition of downstream MAPK and AKT signaling and cell proliferation, and showed anti-tumor activity in HER2 expressing tumor cells. In vivo, tucatinib inhibited the growth of HER2 expressing tumors. The combination of tucatinib and trastuzumab showed increased anti-tumor activity in vitro and in vivo compared to either drug alone.
References
[1] PATRICE A LEE. In VivoActivity of ARRY-380, a Potent, Small Molecule Inhibitor of ErbB2 in Combination with Trastuzumab, Docetaxel or Bevacizumab.[J]. Cancer research, 2009, 69 1: 5104-5104. DOI:10.1158/0008-5472.SABCS-09-5104.
[2] S. MOULDER. Abstract A143: ARRY-380, a selective HER2 inhibitor: From drug design to clinical evaluation.[J]. Molecular Cancer Therapeutics, 2011, 10 1. DOI:10.1158/1535-7163.TARG-11-A143.
[3] V. DINKEL. Abstract 852: ARRY-380, a potent, small molecule inhibitor of ErbB2, increases survival in intracranial ErbB2+ xenograft models in mice[J]. Cancer research, 2012, 72 1: 852-852. DOI:10.1158/1538-7445.AM2012-852.
[4] KOCIE?SKI P. Synthesis of Tucatinib[J]. Synfacts, 2019, 24 1: 0965. DOI:10.1055/s-0039-1690496.
[5] RASHMI K MURTHY. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer.[J]. New England Journal of Medicine, 2020, 382 7: 597-609. DOI:10.1056/NEJMoa1914609.
[6] RASHMI MURTHY. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study.[J]. Lancet Oncology, 2018: 880-888. DOI:10.1016/S1470-2045(18)30256-0.
Properties of Tucatinib
| Density | 1.41±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMF:1.0(Max Conc. mg/mL);2.08(Max Conc. mM) DMSO:49.0(Max Conc. mg/mL);101.97(Max Conc. mM) DMSO:PBS (pH 7.2) (1:2):0.33(Max Conc. mg/mL);0.69(Max Conc. mM) |
| form | A crystalline solid |
| pka | 6.61±0.70(Predicted) |
| color | White to yellow |
Safety information for Tucatinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Tucatinib
| InChIKey | SDEAXTCZPQIFQM-UHFFFAOYSA-N |
| SMILES | N1=C2C(C=C(NC3=NC(C)(C)CO3)C=C2)=C(NC2=CC=C(OC3C=CN4N=CN=C4C=3)C(C)=C2)N=C1 |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 937263-43-9 Tucatinib 98%View Details
937263-43-9 Tucatinib 98%View Details
937263-43-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8