Iopanoic acid
Synonym(s):2-(3-Amino-2,4,6-triiodobenzyl)butyric acid;3-(3-Amino-2,4,6-triiodophenyl)-2-ethylpropanoic acid;Iodopanoic acid
- CAS NO.:96-83-3
- Empirical Formula: C11H12I3NO2
- Molecular Weight: 570.93
- MDL number: MFCD00038687
- EINECS: 202-539-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
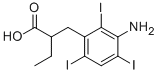
What is Iopanoic acid ?
Chemical properties
Light Brown Solid
Originator
Telepaque,Winthrop,US,1952
The Uses of Iopanoic acid
antiulcer
The Uses of Iopanoic acid
Iodopanoic Acid is an iodine-containing radiocontrast medium used in cholecystography. Iodopanoic Acid is a potent inhibitor of thyroid hormone release from thyroid gland.
Background
Iopanoic acid contains iodine and is useful as a contrast medium in cholecystography.
Definition
ChEBI: Iopanoic acid is a monocarboxylic acid.
Manufacturing Process
(A) Preparation of α-Ethyl-m-Nitrocinnamic Acid: This acid is prepared from
100 g of m-nitrobenzaldehyde, 210 g of butyric anhydride and 73 g of sodium
butyrate. The crude α-ethyl-m-nitrocinnamicacid is crystallized from ethanol
giving about 105 g, MP 140° to 142°C. From the filtrates there may be
isolated a small amount of a stereoisomer, which when pure melts at 105° to
106°C.
(B) Preparation of m-Amino-α-Ethylhydrocinnamic Acid: A mixture of 50 g of
α-ethyl-m-nitrocinnamic acid, 9.1 g of sodium hydroxide, 600 cc of water and
5 teaspoons of Raney nickel catalyst is shaken at 32°C in an atmosphere ofhydrogen at an initial pressure of 450 psi until the calculated amount of
hydrogen is absorbed. The filtered solution is acidified with hydrochloric acid,
made basic with ammonium hydroxide and again acidified with acetic acid.
Upon concentration of this solution, an oil separates which crystallizes upon
standing, giving about 20 g, MP 60° to 68°C. Complete evaporation of the
filtrate and extraction of the residue of inorganic salts with ether gives about
20 g of additional material, MP 54° to 59°C. Recrystallization of the combined
product from benzene petroleum ether gives about 35 g of m-amino-α-
ethylhydrocinnamic acid, MP 67° to 70°C.
(C) Preparation of β-(3-Amino-2,4,6-Triiodophenyll-α-Ethylpropionic Acid: A
solution of 5.0 g of m-amino-α-ethylhydrocinnamic acid in 100 cc of water
containing 5 cc of concentrated hydrochloric acid is added over a period of ?
hour to a stirred solution of 3.2 cc of iodine monochloride in 25 cc of water
and 25 cc of concentrated hydrochloric acid heated to 60°C. After addition is
complete, the heating is continued for one hour longer at 60° to 70°C. A black
oil separates which gradually solidifies.
The mixture is then cooled and sodium bisulfite added to decolorize.
Recrystallization of the product from methanol gives about 8 g, MP 147° to
150°C. The β-(3-amino-2,4,6-triiodophenyl)-α-ethylpropionic acid may be
purified further by precipitation of the morpholine salt from ether solution and
regeneration of the free amino acid by treatment of a methanol solution of the
morpholine salt with sulfur dioxide. The pure amino acid has the MP 155° to
156.5°C.
Therapeutic Function
Diagnostic aid (radiopaque medium)
Metabolism
Not Available
Properties of Iopanoic acid
| Melting point: | 153 °C |
| Boiling point: | 529.1±50.0 °C(Predicted) |
| Density | 2.2567 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO, Methanol |
| form | neat |
| pka | 4.8(at 25℃) |
| form | Solid |
| color | Light Brown |
| Water Solubility | 348.3mg/L(37 ºC) |
| Merck | 14,5058 |
| CAS DataBase Reference | 96-83-3(CAS DataBase Reference) |
| EPA Substance Registry System | Benzenepropanoic acid, 3-amino-.alpha.-ethyl-2,4,6-triiodo- (96-83-3) |
Safety information for Iopanoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Iopanoic acid
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 Iopanoic acid 95% CAS 96-83-3View Details
Iopanoic acid 95% CAS 96-83-3View Details
96-83-3 -
 Iopanoic Acid CAS 96-83-3View Details
Iopanoic Acid CAS 96-83-3View Details
96-83-3 -
 Iopanoic acid CAS 96-83-3View Details
Iopanoic acid CAS 96-83-3View Details
96-83-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4