β-Alanine
Synonym(s):β-Ala;β-Alanine;3-Aminopropionic acid;3-Aminopropionic acid-15N;Beta-alanine
- CAS NO.:107-95-9
- Empirical Formula: C3H7NO2
- Molecular Weight: 89.09
- MDL number: MFCD00008200
- EINECS: 203-536-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
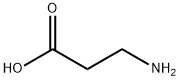
What is β-Alanine?
Description
β-Alanine (or beta-alanine) is a naturally occurring beta amino acid, which is an amino acid in which the amino group is at the β- position from the carboxylate group (i.e., two atoms away. The IUPAC name for β-alanine is 3-amino propanoic acid. Unlike its counterpart α-alanine, β-alanine has no stereocenter.
β-Alanine is not used in the biosynthesis of any major proteins or enzymes. It is formed in vivo by the degradation of dihydrouracil and carnosine. It is a component of the naturally occurring peptides carnosine and anserine and also of pantothenic acid (vitamin B5), which itself is a component of coenzyme A. Under normal conditions, β-alanine is metabolized into acetic acid.
β-Alanine is the rate-limiting precursor of carnosine, which is to say carnosine levels are limited by the amount of available β-alanine. Supplementation with β-alanine has been shown to increase the concentration of carnosine in muscles, decrease fatigue in athletes and increase total muscular work done.
Typically, studies have used supplementing strategies of multiple doses of 400 mg or 800 mg, administered at regular intervals for up to eight hours, over periods ranging from 4 to 10 weeks . After a 10 - week supplementing strategy, the reported increase in intramuscular carnosine content was an average of 80.1% (range 18 to 205%).
Chemical properties
White crystalline powder
Chemical properties
This is a secondary amino acid, which is formed in vivo by the degradation of dihydrouracil and carnosine. Because neuronal uptake and neuronal receptor sensitivity to β-alanine have been demonstrated, the compound may be a false transmitter replacing GABA. A rare genetic disorder, hyper-β-alaninemia, has been reported. It is used as a flavor enhancer, flavoring agent, nutrient supplement or adjuvant. β-Alanine has a slightly sweet taste.
Occurrence
Reported to occur as a component in amino acids; carnosine, anserine, pantothenic acid
The Uses of β-Alanine
β-Alanine has been used as a ligand for the orphan MAS-related receptor. It has also been used in culture media used for certain strains of yeast to test for β-alanine auxotrophy.
The Uses of β-Alanine
β-Alanine is a naturally occurring beta amino acid. β-Alanine is formed in vivo by the degradation of dihydrouracil (D449990) and carnosine. β-Alanine is also the rate-limiting precursor of carnosine, as a result supplementation with β-alanine increases the concentration of carnosine in muscles.
The Uses of β-Alanine
Effective catalyst for the Knoevenagel condensation. Beta Alanine is used as nutrition supplements in food production. Increase muscular strength & power output, Increases Muscle Mass, increase Anaerobic Endurance.
What are the applications of Application
β-Alanine is an endogenous glycine agonist and GABA transporter inhibitor
Definition
ChEBI: A naturally-occurring beta-amino acid comprising propionic acid with the amino group in the 3-position.
Preparation
By heating acrylic acid with concentrated aqueous ammonia under pressure, by addition of acrylonitrile to phthalimide or to ammonia; from β-aminopropionitrile, from succinimide by the Hofmann degradation.
General Description
An N-blocked form of alanine.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
β-Alanine, a β?amino acid, is a component of pantothenic acid and the rate-limiting amino acid in the biosynthesis of the histidinyl antioxidant dipeptides carnosine and anserine. Endogenous β-amino acid that is a nonselective agonist at glycine receptors and a ligand for the G protein-coupled orphan receptor, TGR7 (MrgD). β-Alanine flux plays a cytoprotective role by supporting the osmotic stability of marine organisms, preimplantation mouse embryos and mammalian cells exposed to hypoxic stress.
Purification Methods
Crystallise β-alanine by dissolving it in a hot saturated aqueous solution, filtering, adding four volumes of absolute EtOH and cooling in an ice-bath. Recrystallise it in the same way and then finally, crystallise it from a warm saturated solution in 50% EtOH and adding four volumes of absolute EtOH with cooling in an ice-bath. The crystals are dried in a vacuum desiccator over P2O5. [Donovan & Kegeles J Am Chem Soc 83 255 1961, Beilstein 4 IV 2526.]
Properties of β-Alanine
| Melting point: | 202 °C (dec.)(lit.) |
| Boiling point: | 237.1±23.0 °C(Predicted) |
| Density | 1,437 g/cm3 |
| refractive index | 1.4650 (estimate) |
| FEMA | 3252 | BETA-ALANINE |
| Flash point: | 204-206°C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Crystalline Powder |
| pka | 3.55(at 25℃) |
| color | White |
| PH | 6.0-7.5 |
| Odor | at 100.00 %. odorless |
| Water Solubility | Soluble in water(550g/L). Slightly soluble in alcohol. Insoluble in ether and acetone. |
| Decomposition | 204-206 ºC |
| λmax | λ: 260 nm Amax: ≤0.02 λ: 280 nm Amax: ≤0.02 |
| JECFA Number | 1418 |
| Merck | 14,205 |
| BRN | 906793 |
| Stability: | Stable. Keep dry. |
| CAS DataBase Reference | 107-95-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Beta-alanine(107-95-9) |
| EPA Substance Registry System | .beta.-Alanine (107-95-9) |
Safety information for β-Alanine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for β-Alanine
| InChIKey | UCMIRNVEIXFBKS-UHFFFAOYSA-N |
β-Alanine manufacturer
JSK Chemicals
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 107-95-9 98%View Details
107-95-9 98%View Details
107-95-9 -
 Beta-Alanine 99%View Details
Beta-Alanine 99%View Details -
 ß-Alanine extrapure CHR CAS 107-95-9View Details
ß-Alanine extrapure CHR CAS 107-95-9View Details
107-95-9 -
 beta-Alanine 99% CAS 107-95-9View Details
beta-Alanine 99% CAS 107-95-9View Details
107-95-9 -
 Beta-Alanine CAS 107-95-9View Details
Beta-Alanine CAS 107-95-9View Details
107-95-9 -
 Powder Beta-Alanine Cas: 107-95-9View Details
Powder Beta-Alanine Cas: 107-95-9View Details
107-95-9 -
 Beta-Alanine, Packaging Type: Drum, SOLIDView Details
Beta-Alanine, Packaging Type: Drum, SOLIDView Details
107-95-9 -
 Beta Alanine Powder, Packaging Size: 25kgView Details
Beta Alanine Powder, Packaging Size: 25kgView Details
107-95-9
