Ioversol
- CAS NO.:87771-40-2
- Empirical Formula: C18H24I3N3O9
- Molecular Weight: 807.11
- MDL number: MFCD00867959
- EINECS: 618-068-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-21 21:32:25
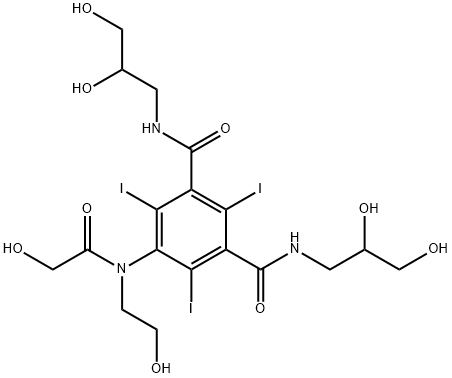
What is Ioversol?
Absorption
Healthy volunteers that received 50 and 150 mL of ioversol, had a corresponding Cmax of 1.45 mg/mL and 2.36 mg/mL and a corresponding tmax of 2.0 min and 4.3 min. AUC was 1.74 mg?hr/mL in healthy volunteers that received 50 mL of ioversol, and 4.43 mg?hr/mL in those that received 150 mL. The pharmacokinetics of ioversol follow an open two compartment model with first-order elimination. Since ioversol is almost completely excreted by the kidney as a parent drug, patients with renal impairment are expected to have a lower elimination rate.
Toxicity
Ioversol overdosage causes life-threatening adverse effects, mainly in the pulmonary and cardiovascular system. In case of an overdose, treatment must focus on the support of vital functions and the prompt institution of symptomatic therapy. The carcinogenic potential of ioversol has not been evaluated, and non-clinical studies suggest that ioversol is not mutagenic and does not affect fertility. The LD50 values in mice and rats are 17 g/kg and 15 g/kg respectively. Developmental toxicity studies were carried out in rats (gestation day 7 to 17) and rabbits (gestation day 6 to 18) given ioversol at the following intravenous doses: 0, 0.2, 0.8, and 3.2 g iodine/kg/day. Adverse effects on embryo-fetal development were not detected in either species, while maternal toxicity was detected in rabbits at 0.8, and 3.2 g iodine/kg/day.
Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use such as ioversol. These serious adverse reactions include death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Caution must be exercised in patients with severely impaired renal function, combined renal and hepatic disease, severe thyrotoxicosis, myelomatosis, or anuria, particularly when large doses are administered.
Chemical properties
White Solid
The Uses of Ioversol
Loversol is a radiopaque contrast agent. Ioversol contains iodine, a substance that absorbs x-rays. Contrast agents are used to allow blood vessels, organs, and other non-bony tissues to be seen more clearly on a CT scan or other radiologic (x-ray) examination.
The Uses of Ioversol
Nonionic, low osmolality, radiographic contrast agent. Diagnostic aid (radiopaque medium).
Background
Ioversol is a non-ionic compound with a tri-iodinated benzene ring used as a contrast dye in diagnostic procedures to visualize different types of organs and tissues. Iodine has a high atomic density, which gives it the ability to attenuate X-rays. The intravascular administration of iodine compounds, such as ioversol, enhances the contrast between vessels in the path of the flow of the contrast medium and normal tissue, allowing the visualization of internal structures. Ioversol is a highly hydrophilic agent considered to be generally safe; however, serious adverse reactions have been reported due to the inadvertent intrathecal administration of ioversol, which is only indicated for intra-arterial and intravenous use.
Ioversol was approved by the FDA in 1989 and is currently indicated for computed tomographic (CT) imaging and contrast enhancement in peripheral arteriography, coronary arteriography, and left ventriculography.
Indications
As the product Optiray 300, the intra-arterial use of ioversol is indicated for cerebral arteriography and peripheral arteriography in adults, while its intravenous use is indicated for CT imaging of the head and body, venography, and intravenous excretory urography in adults.
As the product Optiray 320, the intra-arterial use of ioversol is indicated for cerebral arteriography, peripheral arteriography, visceral and renal arteriography, aortography, coronary arteriography, and left ventriculography in adults, and angiocardiography in pediatic patients. The intravenous use of this product is indicated for CT imaging of the head and body, venography, and intravenous excretory urography in adults and CT imaging of the head and body, and intravenous excretory urography in pediatric patients.
As the product Optiray 350, the intra-arterial use of ioversol is indicated for peripheral arteriography coronary arteriography, and left ventriculography in adults, and angiocardiography in pediatic patients. The intravenous use of this product is indicated for CT imaging of the head and body, venography, intravenous excretory urography, and intravenous digital subtraction angiography (IV-DSA) in adults and CT imaging of the head and body, and intravenous excretory urography in pediatric patients.
What are the applications of Application
Ioversol is a radiographic contrast agent
Definition
Loversol is classified as an organoiodine compound and is used as a contrast dye in diagnostic procedures. It contains high levels of iodine in addition to various hydrophilic groups.
brand name
Optiray (Mallinckrodt).
General Description
Ioversol is a low-osmolar, nonionicmonomer with 47% bound iodine content. It is indicated forangiography, excretory urography, ventriculography, andvarious CT procedures.
Pharmacokinetics
The contrast enhancement obtained with ioversol is related to iodine content. Immediately after ioversol is injected, a peak in iodine plasma levels can be detected. However, depending on the organ being imaged, the time to maximum contrast will vary. The time to maximum contrast enhancement will range from the time iodine plasma levels reach their highest concentration to one hour after intravenous bolus administration. The delay between a peak in iodine plasma levels and maximum contrast enhancement is partly due to the accumulation of iodine-containing medium within a lesion and outside the blood pool.
For angiographies, the maximum contrast enhancement of ioversol occurs almost immediately (15-120 seconds). Following rapid intravenous injection, ioversol can be visualized in the renal parenchyma within 30 to 60 seconds. In patients with normal renal function, opacification of the calyces and pelves takes place within 1-3 minutes, with an optimum contrast within 5-15 minutes.
Side Effects
The most common reaction is nausea, occurring at a rate of 1 percent.
Serious adverse reactions have been reported during post-approval use of Optiray. These serious adverse reactions include but are not limited to: anaphylactic reactions, arrhythmias, temporary blindness, tongue edema, seizures, respiratory arrest, bronchospasm, laryngeal spasm and thrombosis.
Metabolism
Ioversol does not undergo significant metabolism, deiodination or biotransformation.
Properties of Ioversol
| Melting point: | 180-182°C |
| Boiling point: | 864.9±65.0 °C(Predicted) |
| Density | d37 1.371 (c = 320 mgI/ml) |
| storage temp. | Refrigerator |
| solubility | DMSO (Sparingly), Methanol (Slightly) |
| pka | 11.34±0.46(Predicted) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 87771-40-2(CAS DataBase Reference) |
Safety information for Ioversol
Computed Descriptors for Ioversol
Ioversol manufacturer
Related products of tetrahydrofuran
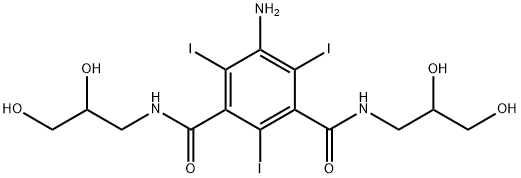
You may like
-
 Ioversol 98%View Details
Ioversol 98%View Details -
 Ioversol >98%View Details
Ioversol >98%View Details -
 Ioversol 98% (HPLC) CAS 87771-40-2View Details
Ioversol 98% (HPLC) CAS 87771-40-2View Details
87771-40-2 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 694-48-4 99%View Details
694-48-4 99%View Details
694-48-4 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3