Hydroxylamine sulfate
Synonym(s):Hydroxylamine sulfate;Hydroxylammonium sulfate
- CAS NO.:10039-54-0
- Empirical Formula: H2O4S.2H3NO
- Molecular Weight: 164.14
- MDL number: MFCD00044869
- EINECS: 233-118-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
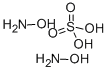
What is Hydroxylamine sulfate?
Chemical properties
Also hydroxylammonium sulfate, or HS, (NH2OH)2·H2S04 is colorless crystals that are soluble in water and slightly soluble in alcohol. The solution has a corrosive action on the skin. Used as a reducing agent, photographic developer, purification agent for aldehydes and ketones, chemical synthesis, textile chemical, oxidation inhibitor for fatty acids, catalyst, in biological and biochemical research, for making oximes for paints and varnishes, and rustproofing.
Physical properties
Colorless, crystalline solid; melts at 177°C (decomposes); very soluble in386 HYDROXYLAMINE HYDROCHLORIDE / HYDROXYLAMINE SULFATEpp-03-25-new dots.qxd 10/23/02 2:38 PM Page 386 water; slightly soluble in alcohol.
The Uses of Hydroxylamine sulfate
Hydroxylamine sulfate may be used to prepare highly sensitive cellulose tape, used for the detection of formaldehyde gas. It may be used in the quantitative determination of perchlorate in biological fluids by spectrophotometric methods .
The Uses of Hydroxylamine sulfate
Used as to purify aldehydes and ketones; reagent for mercury and silver detection in water; reducing agent.
The Uses of Hydroxylamine sulfate
As reducing agent in photography; in synthetic and analytical chemistry; to purify aldehydes and ketones. As antioxidant for fatty acids and soaps. As dehairing agent for hides.
The Uses of Hydroxylamine sulfate
Hydroxylammonium sulfate is a reducing agent in photography; catalyst, swelling agent, and copolymerization inhibitor in polymerization processes; in chemical synthesis; as a textile chemieal; as an oxidation inhibitor; in making oximes for paints and varnishes; in rustproofing; in nondiscoloring short -stoppers for synthetic rubbers; for unhairing hides; in biological and biochemical research; as a purification agent for aldehydes and ketones; converts aldehydes and ketones to oximes and acid chlorides to hydroxamic acids.
Preparation
Hydroxylamine sulfate may be prepared by mixing stoichiometric amountsof hydroxylamine and sulfuric acid. It also may be prepared by electrolytical-ly reducing an aqueous solution of ammonium sulfate.
General Description
Colorless crystalline solid. Contact may cause severe irritation to skin, eyes, and mucous membranes. May be toxic by ingestion.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sulfuric acid fumes may form in fires [USCG, 1999]. Solid Hydroxylamine sulfate explodes when heated to 170°C., [Chem. Process 26:30(1963)]. Sodium ignites on contact with hydroxylamine. (Mellor, 1940, Vol. 8, 292.)
Hazard
Irritant to tissue.
Health Hazard
Inhalation of dust or ingestion may cause systemic poisoning characterized by cyanosis, methemoglobinemia, convulsions, and coma. Contact with eyes or skin causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Sulfuric acid fumes may form in fires.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by skin contact and intraperitoneal routes. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. Moderately explosive when exposed to heat or by chemical reaction. In the presence of alkalies at elevated temperatures, free hydroxylamine is liberated and may decompose explosively. When heated to decomposition it emits toxic fumes of SOx and NOx. See also AMINES and SULFATES.
Purification Methods
Crystallise it from boiling water (1.6mL/g) by cooling to 0o.
Properties of Hydroxylamine sulfate
| Melting point: | 170 °C (dec.)(lit.) |
| Boiling point: | 56.5℃ |
| Density | 1.86 |
| vapor pressure | 0.001Pa at 20℃ |
| storage temp. | -20°C |
| solubility | water: soluble(lit.) |
| form | Crystals |
| color | White |
| PH | 3.6 (10g/l, H2O, 20℃) |
| Water Solubility | 329 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,4828 |
| Stability: | Stable, but may be an explosion hazard - do not heat. May decompose explosively in the presence of alkalies. Air sensitive. Hygroscopic. Incompatible with copper, copper alloys, strong oxidising agents, strong bases, nitrites. |
| CAS DataBase Reference | 10039-54-0(CAS DataBase Reference) |
| EPA Substance Registry System | Hydroxylamine sulfate (2:1) (10039-54-0) |
Safety information for Hydroxylamine sulfate
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Hydroxylamine sulfate
Hydroxylamine sulfate manufacturer
JSK Chemicals
Gujarat State Fertilizers And Chemicals Ltd
ARRAKIS INDUSTRIES LLP
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Hydroxylamine sulfate CAS 10039-54-0View Details
Hydroxylamine sulfate CAS 10039-54-0View Details
10039-54-0 -
 Hydroxylamine sulfate CAS 10039-54-0View Details
Hydroxylamine sulfate CAS 10039-54-0View Details
10039-54-0 -
 Hydroxylamine Sulphate extrapure AR CAS 10039-54-0View Details
Hydroxylamine Sulphate extrapure AR CAS 10039-54-0View Details
10039-54-0 -
 Hydroxylamine sulfate CAS 10039-54-0View Details
Hydroxylamine sulfate CAS 10039-54-0View Details
10039-54-0 -
 Hydroxylammonium sulfate CASView Details
Hydroxylammonium sulfate CASView Details -
 Hydroxylamine Sulphate CASView Details
Hydroxylamine Sulphate CASView Details -
 Hydroxylamine sulfate 99% CAS 10039-54-0View Details
Hydroxylamine sulfate 99% CAS 10039-54-0View Details
10039-54-0 -
 Hydroxylamine Sulphate CASView Details
Hydroxylamine Sulphate CASView Details