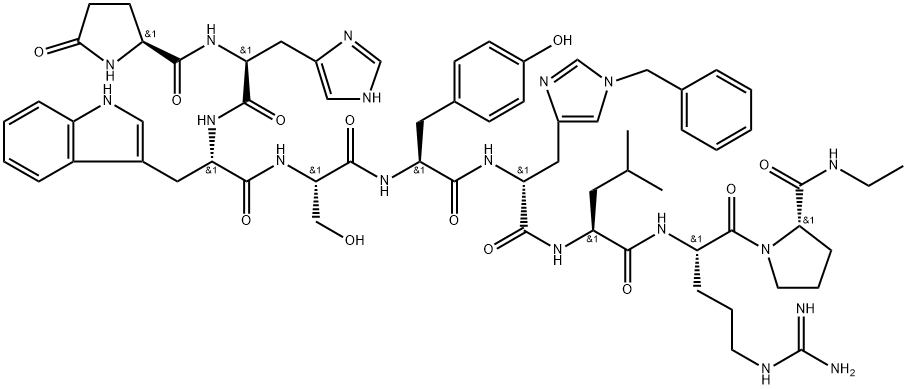
Histrelin
Synonym(s):Histrelin
- CAS NO.:76712-82-8
- Empirical Formula: C66H86N18O12
- Molecular Weight: 1323.53
- MDL number: MFCD00133494
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-23 17:25:29
What is Histrelin?
Absorption
Advanced prostate cancer patients (n = 17) that received a subcutaneous histrelin implant (Vantas, Endo Pharmaceuticals) had peak serum concentrations of 1.10 ± 0.375 ng/mL (mean ± SD) at 12 hours. The continuous subcutaneous release of the histrelin implant was confirmed, as serum levels were sustained throughout the 52-week dosing period. At the end of the 52-week period, the mean serum histrelin concentration was 0.13 ± 0.065 ng/mL. In patients that received a second implant at the end of the 52-week period, the serum histrelin concentration in the first eight weeks was similar to the one detected with the first implant. On average, the residual drug content of 41 histrelin implants (Vantas, Endo Pharmaceuticals) was 56.7 ± 7.71 mcg/day over 52 weeks. Compared to healthy male volunteers that received a subcutaneous bolus dose, the relative bioavailability of histrelin in patients with prostate cancer and normal renal and hepatic function was 92%.
In children with central precocious puberty (CPP, n=47) that received a subcutaneous histrelin implant (Supprelin LA, Endo Pharmaceuticals), the median maximum serum histrelin concentration over the study period was 0.43 ng/mL, which is expected to maintain gonadotropins at prepubertal levels. There were no pharmacokinetic differences between patients previously treated with luteinizing hormone-releasing hormone (LHRH) agonists and those that had not. Food-drug interaction studies have not been performed for histrelin products. Serum histrelin concentrations are 50% higher in prostate cancer patients with mild to severe renal impairment compared to those with no renal or hepatic impairment; however, this difference is not considered clinically relevant.
Toxicity
There were no signs of systemic toxicity in animals injected with up to 200 mcg/kg (rats, rabbits), or 2000 mcg/kg (mice) of histrelin acetate. These concentrations represent 20 to 200 times the maximal recommended human dose of 10 mcg/kg/day. Patients receiving one, two or four histrelin implants (Vantas, Endo Pharmaceuticals) had similar adverse event profiles.
No overdose cases were reported in the clinical trials of the histrelin product Supprelin LA (Vantas, Endo Pharmaceuticals). The administration of high doses of histrelin in animal studies was associated with the expected pharmacological effects. Since both products of histrelin are administered using implants that deliver a constant dose, accidental or intentional overdose is unlikely.
Description
Histrelin, a synthetic gonadotrophin-releasing hormone (GnRH) agonist, was introduced as a first-line therapy for the treatment of central precocious puberty. When administered over a prolonged period, it suppresses the release of gonadotrophin, inhibits ovarian and testicular steroidogenesis, and prevents sexual maturation. Superior to the traditional therapy with progestational agents, histrelin appears to have decelerating effects on skeletal maturation allowing more statural growth and significantly increased final adult height. Histrelin has also been used to treat other disorders including endometriosis, polycystic ovarian disease, uterine leiomyomas, severe premenstrual syndrome and to prevent acute intermittent porphyria.
Originator
Johnson & Johnson (U.S.A.)
The Uses of Histrelin
LHRH agonist.
The Uses of Histrelin
[des-Gly10, D-His(Bzl)6]-LH-RH ethylamide has been used:
- for inducing Batrachoseps gravid females to oviposit
- for comparing the effect of LHRH (luteinizing hormone-releasing hormone) agonists and dopamine antagonists in Lithobates pipiens
- for inducing oviposition in females of Taricha granulosa and Ensatina eschscoltzii
- to induce spawning in Spea hammondii adults
Background
Histrelin is a gonadotropin-releasing hormone (GnRH) agonist that acts as a potent inhibitor of gonadotropin when administered as an implant delivering continuous therapeutic doses. This drug is a synthetic analog of naturally occurring GnRH with a higher potency. Histrelin implants are non-biodegradable, diffusion-controlled, hydrogel polymer reservoirs containing histrelin acetate that need to be replaced every 52 weeks.
Initially, histrelin implants were developed to reduce testosterone to castration levels in patients with advanced prostate cancer. The Vantas product was approved by the FDA in October 2004 for the palliative treatment of this condition. Vantas was later discontinued by Endo Pharmaceuticals Inc. on September 21, 2021.
GnRH agonists are the first line of treatment for children with central precocious puberty (CPP) due to their capacity to reduce LH levels and the concentration of sex steroids. As the product Supprelin LA, histrelin is indicated for the treatment of CPP in children (approved by the FDA in May 2007).
Indications
As the product Supprelin LA (FDA), histrelin is indicated for the treatment of children with central precocious puberty (CPP). As the product Vantas (FDA), histrelin is indicated for the palliative treatment of advanced prostate cancer.
Definition
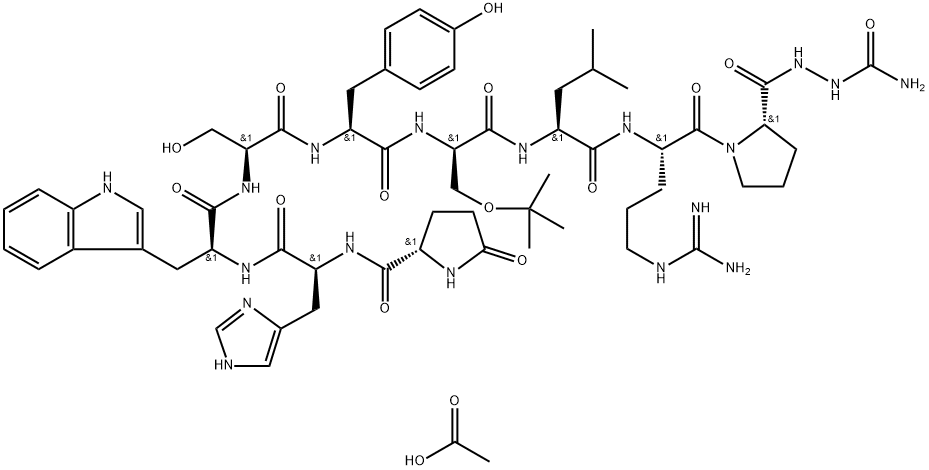
ChEBI: An oligopeptide comprising pyroglutamyl, histidyl, tryptophyl, seryl, tyrosyl, 1-benzyl-D-histidyl, leucyl, arginyl, and N-ethylprolinamide residues joined in sequence. It is a synthetic nonapeptide analogue of gonadotro in-releasing hormone, and is used as a subcutaneous hydrogel implant (particularly as the diacetate salt) for the treatment of prostate cancer and for the suppression of gonadal sex hormone production in children with central precocious puberty.
brand name
Supprelin (Roberts Pharmaceutical).
Biochem/physiol Actions
Glp-His-Trp-Ser-Tyr-His(Bzl)-Leu-Arg-Pro-NHEt ([des-Gly10, D-His(Bzl)6]-LH-RH) is a GnRH (gonadotropin-releasing hormone) agonist, histerelin. GnRH (Glp-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2), which is also referred as LHRH (luteinizing hormone-releasing hormone) or gonadorelin, is crucial for mammalian reproduction and is released from hypothalamic neurons. It is responsible for the secretion of gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), from the pituitary glands. Mutations in GnRH are associated with normosmic congenital hypogonadotropic hypogonadism. Histerelin is used for the treatment of central precocious puberty in children. It inhibits actions of sex steroids on the male and female reproductive organs.
Pharmacokinetics
Histrelin inhibits gonadotropin secretion through the reversible down-regulation of gonadotropin-releasing hormone (GnRH) receptors in the pituitary gland and desensitization of the pituitary gonadotropes. In pediatric patients with central precocious puberty (CPP), long-term treatment with histrelin acetate suppresses the luteinizing hormone (LH) response to GnRH, causing LH levels to decrease to prepubertal levels within one month of treatment. This reduces ovarian and testicular steroidogenesis and slows down linear growth velocity, improving the chance of attaining predicted adult height. When given orally, histrelin acetate is not active.
Both histrelin products (Vantas and Supprelin LA from Endo Pharmaceuticals) cause a transient increase in serum concentrations of estradiol in females and testosterone in both sexes during the first week of treatment. Laboratory tests are also recommended in order to monitor hormone levels. For pediatric patients with central precocious puberty (CPP) using histrelin (Supprelin LA, Endo Pharmaceuticals), LH, follicle-stimulating hormone and estradiol or testosterone should be monitored. In patients with advanced prostate cancer using histrelin (Vantas, Endo Pharmaceuticals), testosterone and prostate-specific antigen should be measured periodically. Issues such as breakage during insertion and difficulty locating and removing implants have been reported.
The Supprelin LA (Endo Pharmaceuticals) product label alerts users about psychiatric events, convulsions and cases of pseudotumor cerebri (idiopathic intracranial hypertension) that have been reported in patients receiving GnRH agonists. The Vantas (Endo Pharmaceuticals) product label alerts users about cases of spinal cord compression and urinary tract obstruction, and an increased risk of hyperglycemia/diabetes and cardiovascular disease in men receiving GnRH agonists.
Metabolism
As a synthetic peptide, histrelin is expected to be metabolized by proteases throughout the body. This will likely result in several peptide fragments produced by hydrolysis. In an in vitro drug metabolism study using human hepatocytes, a single histrelin metabolite resulting from C-terminal dealkylation was identified.
Properties of Histrelin
| alpha | D20 -33.9° (c = 1 in acetic acid) |
| Density | 1.46±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Aqueous Acid (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | powder |
| pka | 9.82±0.15(Predicted) |
| Stability: | Hygroscopic |
Safety information for Histrelin
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Histrelin
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
![[des-Gly10, D-His(Bzl)6]-LH-RH ethylamide CAS 76712-82-8](https://img.chemicalbook.in//Content/image/CP5.jpg) [des-Gly10, D-His(Bzl)6]-LH-RH ethylamide CAS 76712-82-8View Details
[des-Gly10, D-His(Bzl)6]-LH-RH ethylamide CAS 76712-82-8View Details
76712-82-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
