Vinyl acetate
Synonym(s):Acetic acid vinyl ester;Acetoxyethylene;VAc;Vinyl acetate
- CAS NO.:108-05-4
- Empirical Formula: C4H6O2
- Molecular Weight: 86.09
- MDL number: MFCD00008713
- EINECS: 203-545-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:05
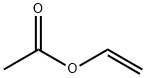
What is Vinyl acetate?
Description
Vinyl acetate monomer (VAM) is a colourless liquid, immiscible or slightly soluble in water. VAM is a flammable liquid. VAM has a sweet, fruity smell (in small quantities), with sharp, irritating odour at higher levels. VAM is an essential chemical building block used in a wide variety of industrial and consumer products. VAM is a key ingredient in emulsion polymers, resins, and intermediates used in paints, adhesives, coatings, textiles, wire and cable polyethylene compounds, laminated safety glass, packaging, automotive plastic fuel tanks, and acrylic fibres. Vinyl acetate is used to produce polyvinyl acetate emulsions and resins. Very small residual levels of vinyl acetate have been found present in products manufactured using VAM, such as moulded plastic items, adhesives, paints, food packaging containers, and hairspray.
Description
Vinyl acetate is an important industrial monomer that is used to make homopolymers and copolymers with a wide variety of applications. It is a volatile, flammable liquid that, like many esters, has a pleasant, fruity aroma.
Esters are usually synthesized from an alcohol and an acid; but in the case of vinyl acetate, the starting alcohol would be vinyl alcohol, which is not stable. To learn how vinyl acetate is made, see “Vinyl acetate synthesis and current production”.
The homopolymer poly(vinyl acetate)1 (PVA or PVAc) is an important polymer used in adhesives, textile sizing, and even chewing gum. In 1912, German chemist Fritz Klatte discovered PVA when he observed that vinyl acetate easily reacts with itself. Klatte’s PVA formulations were dense solids; but over the years, researchers developed methods for producing the polymer in other forms.
One of the most common forms of PVA is made by aqueous free-radical emulsion polymerization. Water, a surfactant, and an initiator (e.g., sodium persulfate) are mixed and heated. Vinyl acetate monomer (frequently called VAM in the industry) is added slowly, and the system is heated until the reaction is complete.2 The product is a milklike latex emulsion.
PVA latices are widely used in adhesives. One of the most familiar is Elmer’s Glue. PVA adhesives are used in packaging applications such as shipping boxes and bags, food containers, envelopes, tapes, and as binders for paper, plastics, and foils.
In addition to making homopolymers, vinyl acetate is combined with other monomers such as ethylene and acrylates to produce a variety of copolymers. Ethylene–vinyl acetate copolymers (EVAs) can be made in bulk or by emulsion polymerization. Emulsion EVAs have many uses, including adhesives that are similar to vinyl acetate homopolymers. Bulk polymer applications range from large-volume industrial uses to the handy glue sticks you may have at home or in school.
Vinyl acetate–acrylate emulsion copolymers (“acrylics”) are used primarily in paints and other coatings. They have largely replaced oil-based coatings for economic and environmental reasons.
This year’s National Chemistry Week’s theme, “Sticking with Chemistry”, pays homage to the numerous adhesive polymers prepared from vinyl acetate and other monomers. Be sure to check it out at www.acs.org/ncw.
1. Often incorrectly expressed as polyvinyl acetate.
2. In some cases, all of the PVA is added at the beginning of the process.
Chemical properties
Vinyl acetate is a colorless, flammable liquid with a pungent odor. The odor threshold is 0.12 ppm 0.3 ppm (NY, NJ).it is the precursor to polyvinyl acetate, an important polymer in industry.
Physical properties
Colorless, watery liquid with a pleasant, fruity odor. Experimentally determined detection and recognition odor threshold concentrations were 400 μg/m3 (120 ppbv) and 1.4 mg/m3 (400 ppbv), respectively (Hellman and Small, 1974).
The Uses of Vinyl acetate
Vinyl acetate is primarily used to produce polyvinyl acetate emulsions and polyvinyl alcohol. The principal use of these emulsions has been in adhesives, paints, textiles, and paper products.
The Uses of Vinyl acetate
In polymerized form for plastic masses, films and lacquers; in plastic film for food packaging. As modifier for food starch.
What are the applications of Application
Vinyl acetate is a useful building block
Production Methods
Vinyl acetate is an industrial chemical that is produced in large amounts in the United States. The worldwide production capacity of vinyl acetate monomer (VAM) was estimated at 6,154,000 tonnes/annum in 2007, with most capacity concentrated in the United States (1,585,000 all in Texas), China (1,261,000), Japan (725,000) and Taiwan (650,000) . The average list price for 2008 was $1600/tonne. Celanese is the largest producer (ca 25% of the worldwide capacity), while other significant producers include China Petrochemical Corporation (7 %), Chang Chun Group (6%) and LyondellBasell (5%).
It is a key ingredient in furniture-glue.
Preparation
The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst.
Ethylene + acetic acid + 1/2 O2 → Vinyl acetate +H2O
But by products are also generated:
Ethylene + 3 O2 → 2CO2 + 2H2O
Vinyl acetate is also prepared by the gas-phase addition of acetic acid to acetylene.
Definition
ChEBI: Vinyl acetate is an acetate ester.
Reactions
Vinyl acetate undergoes many of the reactions anticipated for an alkene and an ester. Bromine adds to give the dibromide. Hydrogen halides add to give 1-haloethyl acetates, which cannot be generated by other methods because of the non - availability of the corresponding halo-alcohols. Acetic acid adds in the presence of palladium catalysts to give ethylidene diacetate, CH3CH(OAc)2. It undergoes transesterification with a variety of carboxylic acids.The alkene also undergoes Diels - Alder and 2 + 2 cycloadditions.
General Description
Vinyl acetate appears as a clear colorless liquid. Flash point 18 °F. Density 7.8 lb / gal. Slightly soluble in water. Vapors are heavier than air. Vapors irritate the eyes and respiratory system. May polymerize if heated or contaminated. If polymerization occurs inside a container, the container may violently rupture. Used to make adhesives, paints, and plastics.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Vinyl acetate may undergo spontaneous exothermic polymerization on exposure to light. Reacts with air or water to produces peroxides that initiate explosively violent polymerization. Reacts with hydrogen peroxide to form explosive peracetic acid. Reacts with oxygen to form explosive peroxides. Forms explosive Vinyl acetate ozonide on contact with ozone. Undergoes violent or explosive reactions with 2-aminoethanol, chlorosulfonic acid, ethylenediamine, mineral acids (hydrochloric acid, hydrofluoric acid, nitric acid, sulfuric acid, oleum), and peroxides [Lewis, 3rd ed., 1993, p. 1311]. Polymerization initiated by dibenzoyl peroxide in ethyl acetate accelerated out of control, ignited and exploded [Vervalin, 1973, p. 81]. Polymerization in toluene solution has caused several large industrial explosions [MCA Case History No. 2087].
Health Hazard
Vinyl acetate has been related to reproductive abnormalities. It is a skin and upper respiratory tract irritantand a central nervous system depressant. Exposure caused gradual deterioration of heart muscles.
Fire Hazard
When heated to decomposition, Vinyl acetate burns and emits acrid fumes. Highly dangerous when exposed to heat, flames or oxidizers; explosion hazard with strong acids and strong oxidizers. Incompatible with alumina, oxidizing materials, 2-aminoethanol, chlorosulfonic acid; ethyleneimine; 36% hydrochloric acid; 48.7% hydrofluoric acid; 70% nitric acid; oleum; 96% sulfuric acid; ethylene diamine; peroxides and silica gel. Avoid light or any polymerizing initiator. Hazardous polymerization can be initiated by organic and inorganic peroxides; azo compounds; redox systems (including organometallic components); light; and high energy radiation.
Flammability and Explosibility
Highly flammable
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. Moderately toxic by ingestion, inhalation, and intraperitoneal routes. A skin and eye irritant. Experimental reproductive effects. Human mutation data reported. Highly dangerous fire hazard when exposed to heat, flame, or oxidzers. A storage hazard, it may undergo spontaneous exothermic polymerization. Reaction with air or water to form peroxides that catalyze an exothermic polymerization reaction has caused several large industrial explosions. Reaction with hydrogen peroxide forms the explosive peracetic acid. Reacts with oxygen above 50℃ to form an unstable explosive peroxide. Reacts with ozone to form the explosive vinyl acetate ozonide. Solution polymerization of the acetate dmolved in toluene has resulted in large industrial explosions. Polymerization reaction with dibenzoyl peroxide + ethyl acetate may release ignitable and explosive vapors. The vapor may react vigorously with desiccants (e.g., sihca gel or alumina). Incompatible (explosive) with 2-amino ethanol, chlorosulfonic acid, ethylenediamine, ethyleneimine, HCl, HF, HNO3, oleum, peroxides, H2SO4. See also ESTERS.
Potential Exposure
Vinyl acetate is used primarily in polymerization processes to produce polyvinyl acetate; polyvinyl alcohol, and vinyl acetate copolymer. The polymers, usually made as emulsions, suspensions, solutions, or resins, are used to prepare adhesives, paints, paper coatings, and textile finishes. Low molecular weight vinyl acetate is used as a chewing gum base.
Carcinogenicity
There is inadequate evidence in humans for the carcinogenicity of vinyl acetate. There is limited evidence in experimental animals for the carcinogenicity of vinyl acetate. Therefore, IARC has classified vinyl acetate as possibly carcinogenic to humans (Group 2B). This conclusion was based on the following evidence: vinyl acetate is rapidly transformed into acetaldehyde in human blood and animal tissues, there is sufficient evidence in experimental animals for the carcinogenicity of acetaldehyde, both vinyl acetate and acetaldehyde induce nasal cancer in rats after administration by inhalation, and vinyl acetate and acetaldehyde are genotoxic in human cells in vitro and in animals in vivo .
Shipping
UN1301 Vinyl acetate, stabilized, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Inhibitors such as hydroquinone and other impurities are removed by drying with CaCl2 and fractionally distilling under nitrogen, then refluxing briefly with a small amount of benzoyl peroxide and redistilling it under nitrogen. Store it in the dark at 0o. Add inhibitor (~0.004%) for storage. [Beilstein 2 IV 176.]
Polymerization
It can be polymerized, either by itself to make polyvinyl acetate or with other monomers to prepare copolymers such as ethylene-vinyl acetate (EVA), vinyl acetate-acrylic acid (VA / AA), polyvinyl chloride acetate (PVCA), and polyvinylpyrrolidone (Vp / Va Copolymer, used in hair gels). Due to the instability of the radical, attempts to control the polymerization via most 'living/controlled' radical processes have proved problematic. However, RAFT (or more specifically MADIX) polymerization offers a convenient method of controlling the synthesis of PVA by the addition of a xanthate or a dithiocarbamate chain transfer agent.
Toxicity evaluation
On January 31, 2009, the Government of Canada's final assessment concluded that exposure to vinyl acetate is not considered to be harmful to human health . This decision under the Canadian Environmental Protection Act (CEPA) was based on new information received during the public comment period, as well as more recent information from the risk assessment conducted by the European Union.
Incompatibilities
Vinyl acetate may undergo spontaneous exothermic polymerization on exposure to light. Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, strong light and UV. The vapor may react vigorously with silica gel or aluminum, acids, bases, silica gel; alumina, oxidizers, azo compounds. Ozone readily polymerizes in elevated temperatures, under the influence of light, or peroxides. Usually contains a stabilizer to prevent polymerization.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Vinyl acetate
| Melting point: | -93 °C (lit.) |
| Boiling point: | 72-73 °C (lit.) |
| Density | 0.934 g/mL at 25 °C (lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 88 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 20 °F |
| storage temp. | 2-8°C |
| solubility | 20g/l |
| form | Liquid |
| appearance | colorless liquid |
| color | Clear colorless to almost colorless |
| PH | 7 (20g/l, H2O, 20℃) |
| explosive limit | 2.6-13.4%(V) |
| Water Solubility | 23 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,9992 |
| BRN | 1209327 |
| Henry's Law Constant | 4.81 (calculated, Howard, 1989) |
| Exposure limits | NIOSH REL: 15-min ceiling 4 ppm (15 mg/m3); ACGIH TLV: TWA 10 ppm,
STEL 15 ppm (adopted). |
| Dielectric constant | 4.5(23℃) |
| Stability: | Stable. Highly flammable. Incompatible with acids, bases, oxidizing agents, peroxides, chlorosulfonic acid, ethylene imine, hydrochloric acid, oleum, nitric acid, sulfuric acid, 2-aminoethanol, light. Susceptible to polymerization; commercial product may be stabilized by the addition of hydroquinone. |
| CAS DataBase Reference | 108-05-4(CAS DataBase Reference) |
| IARC | 2B (Vol. Sup 7, 63) 1995 |
| NIST Chemistry Reference | Acetic acid ethenyl ester(108-05-4) |
| EPA Substance Registry System | Vinyl acetate (108-05-4) |
Safety information for Vinyl acetate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Vinyl acetate
Vinyl acetate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Vinyl acetate 98%View Details
Vinyl acetate 98%View Details -
 Vinyl acetate 98%View Details
Vinyl acetate 98%View Details -
 Vinyl acetate 108-05-4 98%View Details
Vinyl acetate 108-05-4 98%View Details
108-05-4 -
 Vinyl acetate CAS 108-05-4View Details
Vinyl acetate CAS 108-05-4View Details
108-05-4 -
 Vinyl acetate, 99% CAS 108-05-4View Details
Vinyl acetate, 99% CAS 108-05-4View Details
108-05-4 -
 Vinyl Acetate Monomer CASView Details
Vinyl Acetate Monomer CASView Details -
 Vinyl Acetate CASView Details
Vinyl Acetate CASView Details -
 Acetic Acid Vinyl Ester, For IndustrialView Details
Acetic Acid Vinyl Ester, For IndustrialView Details
108-05-4
