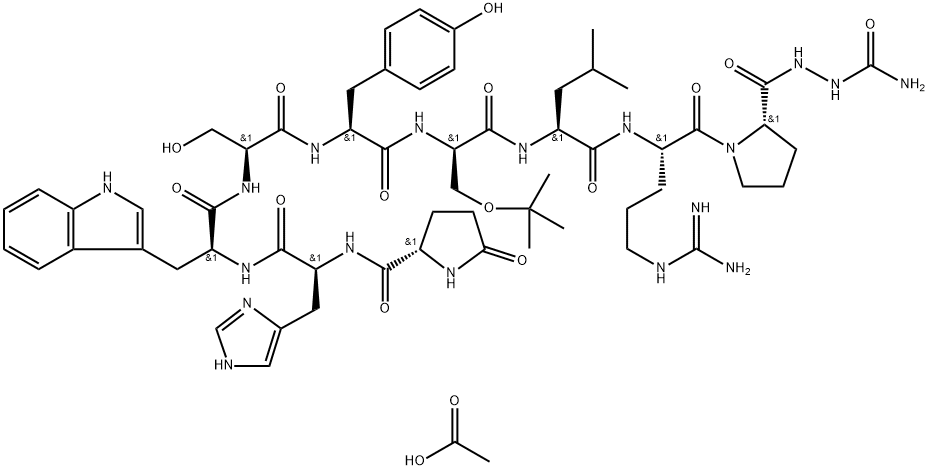
Goserelin acetate
Synonym(s):[D-Ser(tBu)6,Azagly10]-LHRH acetate salt;Goserelin acetate
- CAS NO.:145781-92-6
- Empirical Formula: C61H88N18O16
- Molecular Weight: 1329.49
- MDL number: MFCD00867894
- EINECS: 652-995-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
What is Goserelin acetate?
Description
Goserelin acetate, like leuprolide acetate, is a synthetic superagonist nonapeptide analogue of
GnRH that possesses greater potency than the natural hormone. Note that it contains D-Ser(But)
and NH-NHCONH2 in place of Gly6 and Gly10-NH2, respectively. That is, the C terminal modification simply has an NH substituting for the CH2 of Gly, and like the C-terminal
change in leuprolide acetate, this inhibits enzymatic degradation of the peptide by the
postproline carboxyamide peptidase.
Goserelin acetate is available in the form of a small, solid pellet that is administered as an SC
implant for the palliative treatment of advanced, metastatic breast cancer in pre- and
perimenopausal women or, similarly, as a palliative in advanced prostatic cancer. The rationale
for this drug's use is, as described above, its ability as a superagonist to bring the levels of
estradiol or testosterone to near castrate levels, thus slowing the progression of breast or
prostate carcinoma, respectively. Additionally, goserelin acetate is approved for use in treating
endometriosis for up to 6 months.
Description
Goserelin is a synthetic gonadotropin-releasing hormone (GNRH) agonist that binds to the GNRH receptor (GNRHR; Ki = 1.6 nM, in CHO cells expressing the human receptor). It binds to mouse pituitary αT3-1 cells and human placenta (Kds = 2 and 580 nM, respectively) and increases intracellular calcium in αT3-1 cells. Goserelin induces testosterone production in vitro within 4 h in rat Leydig cells (ED50 = 83 nM) but decreases testosterone plasma level in vivo in rats over a period of 2 to 24 weeks. It inhibits tumor growth in a DU145 human prostate carcinoma mouse xenograft model when administered at a dose of 100 μg per day. Formulations containing goserelin have been used in the treatment of hormone-dependent breast and prostate cancers, as well as endometriosis and uterine fibroids.
Chemical properties
White Solid
The Uses of Goserelin acetate
Synthetic peptide agonist analog of LH-RH. Antineoplastic (hormonal).
The Uses of Goserelin acetate
Anticancer
The Uses of Goserelin acetate
injectable gonadotropin releasing hormone superagonist (GnRH agonist) or luteinizing hormone antiproliferative activity in breast, prostate and endometrial cancers
General Description
Goserelin acetate is a synthetic decapaptide, which is a potent analog of LHRH (luteinizing hormone releasing hormone).
Biochem/physiol Actions
Goserelin acetate peptide results in significant inhibition of gonadotropin release and suppresses steroidogenesis in ovaries and testis. Thus, it leads to a reduction of testosterone to castration levels and estrogen to postmenopausal levels. Therefore, this peptide is used for reducing testosterone levels in patients with locally advanced prostate cancer.
Clinical Use
Goserelin acetate also is used in combination with the antiandrogen flutamide for shrinking
prostate carcinoma before radiation therapy. This maximal androgen blockade combination is
used when the prostate carcinoma has been staged as locally confined to the prostate gland,
with one or both lobes as well as the seminal vesicles involved. The treatment should start 8
weeks before radiation treatment begins and be continued throughout the radiation therapy.
Furthermore, women who are to undergo hysterectomy for menorrhagia can benefit from
previous treatment with goserelin acetate, because it is able to induce endometrial thinning.
This thinning of the endometrium improves the operating environment by causing less
intrauterine bleeding, increased postoperative amenorrhea, and decreased dysmenorrhea
following surgery, which is why goserelin acetate is approved for inducing endometrial thinning
prior to a patient undergoing a hysterectomy for heavy menstrual bleeding.
Properties of Goserelin acetate
| Melting point: | >190°C (dec.) |
| storage temp. | -20°C |
| solubility | H2O: 20 mg/mL, clear, colorless |
| form | white powder |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for Goserelin acetate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Goserelin acetate
| InChIKey | IKDXDQDKCZPQSZ-JHYYTBFNSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 145781-92-6 Goserelin acetate 99%View Details
145781-92-6 Goserelin acetate 99%View Details
145781-92-6 -
 Goserelin acetate CAS 145781-92-6View Details
Goserelin acetate CAS 145781-92-6View Details
145781-92-6 -
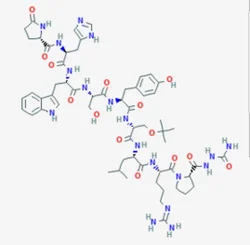 Goserelin AcetateView Details
Goserelin AcetateView Details
65807-02-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
