GUANABENZ ACETATE
Synonym(s):1-(2,6-Dichlorobenzylideneamino)guanidine acetate salt;1-(2,6-Dichlorobenzylideneamino)guanidine, Acetate, WY-8678;Guanabenz Acetate - CAS 23256-50-0 - Calbiochem;Guanabenz acetate salt;WY-8678
- CAS NO.:23256-50-0
- Empirical Formula: C10H12Cl2N4O2
- Molecular Weight: 291.13
- MDL number: MFCD00153801
- EINECS: 245-534-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
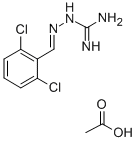
What is GUANABENZ ACETATE?
The Uses of GUANABENZ ACETATE
antihypertensive
The Uses of GUANABENZ ACETATE
α2-adrenergic agonist and IGRS selective ligand
What are the applications of Application
Guanabenz acetate is an α2A-AR agonist and IGRS selective ligand
Definition
ChEBI: Guanabenz acetate is a dichlorobenzene. It has a role as a geroprotector.
brand name
Wytensin (Wyeth).
General Description
Guanabenz acetate, [(2,6-dichlorobenzylidene)amino]guanidine monoacetate (Wytensin), is acentral 2-adrenergic agonist that reduces the release of norepinephrinefrom the neuron when stimulated. The effect ofthe drug results in decreased sympathetic tone in the heart,kidneys, and peripheral blood vessels. The drug does not produceorthostatic hypotension.
Biological Activity
α 2 -adrenergic agonist and IGRS (imidazoline I 2 binding site) selective ligand.
Biochem/physiol Actions
Centrally acting α2 adrenoceptor agonist; I2 imidazoline binding site ligand; antihypertensive.
Pharmacokinetics
The oral bioavailability of guanabenz is 70 to 80%. Following an oral dose, the hypotensive effect of guanabenz begins
within 1 hour, peaks within 2 to 7 hours, and is diminished within 6 to 8 hours. It has an elimination half-life averaging
4 to 14 hours. The blood pressure response can persist for at least 12 hours. Following IV dosing, guanabenz is
distributed into the CNS, with brain concentrations 3 to 70 times higher than concurrent plasma concentrations.
Guanabenz is approximately 90% bound to plasma proteins. In patients with hepatic or renal impairment, its elimination
half-life may be prolonged.
Guanabenz is metabolized principally by hydroxylation to its inactive metabolite, 4-hydroxyguanabenz, which is
eliminated in the urine as its glucuronide (major) and sulfate conjugates. Guanabenz and its inactive
metabolites are excreted principally in urine, with approximately 70 to 80% of its oral dose excreted in urine within 24
hours and approximately 10–30% in feces via enterohepatic cycling. Approximately 40% of an oral dose of guanabenz
is excreted in urine as 4-hydroxyguanabenz and its glucuronide, and less than 5% is excreted unchanged. The
remainder is excreted as unidentified metabolites and their conjugates.
Clinical Use
Overall, the therapeutic applications for guanabenz are similar to those of clonidine and other α2-adrenergic agonists. One advantage for guanabenz is its once-a-day dosing schedule. Guanabenz has been used in diabetic patients with hypertension without adverse effect on the control of or therapy for diabetes, and it has been effective in hypertensive patients with chronic obstructive pulmonary disease, including asthma, chronic bronchitis, or emphysema. Guanabenz has been used alone or in combination with naltrexone in the management of opiate withdrawal in patients physically dependent on opiates and undergoing detoxification. Guanabenz also has been used as an analgesic in a limited number of patients with chronic pain
Side Effects
Overall, the frequency of adverse effects produced by guanabenz is similar to that produced by clonidine and the other α2-adrenergic agonists, but the incidence is lower. As with the other centrally active sympatholytics (e.g., clonidine), abrupt withdrawal of guanabenz may result in rebound hypertension, but the withdrawal syndrome symptoms appear to be less severe.
storage
Store at RT
Properties of GUANABENZ ACETATE
| Melting point: | 227-229℃ (decomposition) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: 11 mg/mL |
| form | solid |
| color | white |
| Merck | 14,4558 |
| Stability: | Hygroscopic |
Safety information for GUANABENZ ACETATE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for GUANABENZ ACETATE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Guanabenz Acetate CAS 23256-50-0View Details
Guanabenz Acetate CAS 23256-50-0View Details
23256-50-0 -
 Guanabenz acetate 96% CAS 23256-50-0View Details
Guanabenz acetate 96% CAS 23256-50-0View Details
23256-50-0 -
 Guanabenz acetate CAS 23256-50-0View Details
Guanabenz acetate CAS 23256-50-0View Details
23256-50-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1