GSK1349572
- CAS NO.:1051375-16-6
- Empirical Formula: C20H19F2N3O5
- Molecular Weight: 419.38
- MDL number: MFCD20488027
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
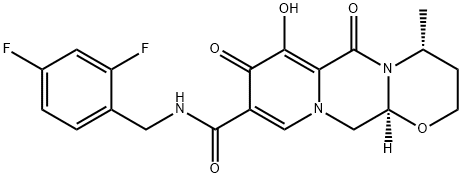
What is GSK1349572?
Absorption
When 50 mg of dolutegravir once daily was orally administered to HIV-1 infected adults, the AUC, Cmax, and Cmin is 53.6 mcg h/mL, 3.67 mcg/mL, and 1.11 mcg/mL, respectively. The peak plasma concentration was observed 2 to 3 hours post-dose. Steady state is achieved within approximately 5 days with average accumulation ratios for AUC, Cmax, and C24h ranging from 1.2 to 1.5. When 50 mg once daily is given to pediatric patients (12 to < 18 years and weighing ≥40 kg) the Cmax, AUC, and C24 is 3.49 mcg/mL, 46 mcg.h/mL, and 0.90 mcg/mL respectively.
Toxicity
Data from an ongoing birth outcome surveillance study has identified an increased risk of neural tube defects when dolutegravir is administered at the time of conception. As defects related to the closure of the neural tube occur from conception through the first 6 weeks of gestation, embryos exposed to dolutegravir from the time of conception through the first 6 weeks of gestation are at potential risk.
Advise adolescents and adults of childbearing potential, including those actively trying to become pregnant, of the potential risk of neural tube defects with the use of TIVICAY and TIVICAY PD. Assess the risks and benefits of TIVICAY and TIVICAY PD and discuss with the patient to determine if an alternative treatment should be considered at the time of conception through the first trimester of pregnancy or if pregnancy is confirmed in the first trimester. A benefit-risk assessment should consider factors such as the feasibility of switching to another antiretroviral regimen, tolerability, ability to maintain viral suppression, and risk of HIV-1 transmission to the infant against the risk of neural tube defects associated with in-utero dolutegravir exposure during critical periods of fetal development [see Warnings and Precautions (5.3)].
There are insufficient human data on the use of dolutegravir during pregnancy to definitively assess a drug-associated risk for birth defects and miscarriage. The background risk for major birth defects for the indicated population is unknown. In the U.S. general population, the estimated background rate for major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
In animal reproduction studies, no evidence of adverse developmental outcomes was observed with dolutegravir at systemic exposures (AUC) less than (rabbits) and approximately 27 times (rats) the exposure in humans at the maximum recommended human dose (MRHD) of TIVICAY (see Data).
There is no known specific treatment for an overdose with TIVICAY or TIVICAY PD. If an overdose occurs, the patient should be monitored, and standard supportive treatment applied as required. As dolutegravir is highly bound to plasma proteins, it is unlikely that it will be significantly removed by dialysis.
Description
In August 2013, the US FDA approved dolutegravir (also referred to as S/GSK1349572) for the treatment of HIV-1 infection in adults and children ages 12 years and older in combination with other antiretroviral drugs. Dolutegravir was approved in Canada in November 2013. HIV/AIDS remains a global epidemic with 35 million people infected, including 2.3 million new infections as of 2012. Dolutegravir joins raltegravir and elvitegravir (this chapter of ARMC) as the latest of three FDA-approved HIV integrase strand transfer inhibitors (INSTIs). Dolutegravir was discovered by rational design from a literature diketo acid HIV integrase inhibitor utilizing X-ray coordinates to predict ideal bond angles between the diketone and distal benzyl group. In dolutegravir, the monocyclic component of the reported inhibitor was replaced with the tricyclic carbamoyl pyridone moiety. The researchers postulated that the appropriate arrangement of three oxygens would permit chelation with two magnesium ions in the binding site thus affording improved potency. Ultimately, this arrangement along with further modifications afforded dolutegravir, a potent inhibitor of HIV integrase (IC50=1.7 nM).
Chemical properties
White Solid
Originator
Shionogi & GlaxoSmithKline (United States)
The Uses of GSK1349572
Dolutegravir is a second generation HIV-1 integrase strand transfer inhibitor. Dolutegravir is currently in Phase III clinical trials for the treatment of HIV infection. Dolutegravir has been shown to potently inhibit HIV replication in cells such as peripheral blood mononuclear cells (PBMCs), MT-4 cells and CIP4 cells infected with a self-inactivating PHIV lentiviral vector.
The Uses of GSK1349572
Dolutegravir is a second generation HIV-1 integrase strand transfer inhibitor. Dolutegravir is currently in Phase III clinical trials for the treatment of HIV infection. Dolutegravir has been shown to potently inhibit HIV replication in cells such as peripheral blood mononuclear cells (PBMCs), MT-4 cells and CIP4 cells infected with a self-inactivating PHIV lentiviral vector.
Background
Dolutegravir is an HIV-1 integrase inhibitor that blocks the strand transfer step of the integration of the viral genome into the host cell (INSTI). The effect of this drug has no homology in human host cells, which gives it excellent tolerability and minimal toxicity. Dolutegravir was developed by ViiV Healthcare and FDA-approved on August 12, 2013. On November 21, 2017, dolutegravir, in combination with rilpivirine, was approved as part of the first complete treatment regimen with only two drugs for the treatment of adults with HIV-1 named Juluca.
Indications
Dolutegravir is indicated in combination with other antiretroviral agents for the treatment of patients with HIV-1 infection that comply with the characteristics of being adults or children aged 12 years and older and present at least a weight of 40 kg. The FDA combination therapy approval of dolutegravir and rilpivirine is indicated for adults with HIV-1 infections whose virus is currently suppressed (< 50 copies/ml) on a stable regimen for at least six months, without history of treatment failure and no known substitutions associated to resistance to any of the two components of the therapy.
Dolutegravir is also available in combination with lamivudine and abacavir for the treatment of adult and pediatric patients with HIV-1 who weigh ≥10kg.
What are the applications of Application
S/GSK1349572 is a next-generation and two-metal-binding HIV-1 integrase inhibitor
Definition
ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of (4R,12aS)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]ox zine-9-carboxylic acid with the amino group of 2,4-difluorobenzylamine. Used (as its sodium salt) for treatment of HIV-1.
brand name
Tivicay
Pharmacokinetics
HIV-1 infected subjects on dolutegravir monotherapy demonstrated rapid and dose-dependent reduction of antiviral activity with declines of HIV-1 RNA copies per ml. The antiviral response was maintained for 3 to 4 days after the last dose. The sustained response obtained in clinical trials indicates that dolutegravir has a tight binding and longer dissociative half-life providing it a high barrier to resistance. The combination therapy (ripivirine and dolutegravir) presented the same viral suppression found in previous three-drug therapies without integrase strand transfer inhibitor mutations or rilpivirine resistance.
Clinical Use
Integrase inhibitor:
Treatment of HIV
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: concentration reduced by St John’s
wort.
Antiepileptics: concentration reduced by
carbamazepine and possibly fosphenytoin,
oxcarbazepine, phenobarbital, phenytoin and
primidone.
Antivirals: concentration reduced by efavirenz,
tipranavir, etravirine and fosamprenavir; possibly
reduced by nevirapine.
Metabolism
Dolutegravir is highly metabolized through three main pathways and it forms no long-lived metabolites. The first pathway is defined by the glucuronidation by UGT1A1, the second pathway by carbon oxidation by CYP3A4 and the third pathway is what appears to be a sequential oxidative defluorination and glutathione conjugation. The main metabolite found in blood plasma is the ether glucuronide form (M2) and its chemical properties disrupt its ability to bind metal ions, therefore, it is inactive.
Metabolism
Dolutegravir is primarily metabolised through
glucuronidation via UGT1A1 with a minor CYP3A
component.
53% of the total oral dose is excreted unchanged in
the faeces. It is unknown if all or part of this is due to
unabsorbed active substance or biliary excretion of the
glucuronidate conjugate, which can be further degraded to
form the parent compound in the gut lumen.
Properties of GSK1349572
| Melting point: | 187-189°C |
| Boiling point: | 669.0±55.0 °C(Predicted) |
| Density | 1.53 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Heated, Sonicated) |
| form | Solid |
| pka | 4.50±1.00(Predicted) |
| color | White to Pale Beige |
Safety information for GSK1349572
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for GSK1349572
GSK1349572 manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethyl-phenyl]amino]pyrimidin-2-yl]amino]benzonitrile](https://img.chemicalbook.in/CAS/GIF/500287-72-9.gif)


You may like
-
 1051375-16-6 99%View Details
1051375-16-6 99%View Details
1051375-16-6 -
 Dolutegravir 98%View Details
Dolutegravir 98%View Details
1051375-16-6 -
 Dolutegravir 1051375-16-6 98%View Details
Dolutegravir 1051375-16-6 98%View Details
1051375-16-6 -
 1051375-16-6 Dolutegravir 99%View Details
1051375-16-6 Dolutegravir 99%View Details
1051375-16-6 -
 1051375-16-6 99%View Details
1051375-16-6 99%View Details
1051375-16-6 -
 Dolutegravir 99%View Details
Dolutegravir 99%View Details -
 Dolutegravir 98% (HPLC) CAS 1051375-16-6View Details
Dolutegravir 98% (HPLC) CAS 1051375-16-6View Details
1051375-16-6 -
 Dolutegravir 95% CAS 1051375-16-6View Details
Dolutegravir 95% CAS 1051375-16-6View Details
1051375-16-6
