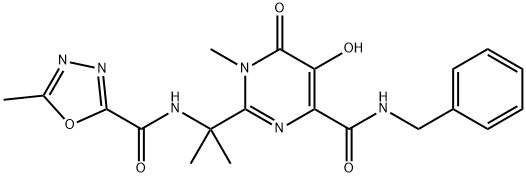
Raltegravir
- CAS NO.:518048-05-0
- Empirical Formula: C20H21FN6O5
- Molecular Weight: 444.42
- MDL number: MFCD10698872
- EINECS: 610-733-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-09-25 20:20:04
What is Raltegravir?
Absorption
Absorbed from the gastrointestinal tract.
Description
Joining maraviroc as a unique approach to battling HIV-1, raltegravir, an inhibitor of HIV-1 integrase, represents the first in its class to be developed and launched as a combination treatment with other antiretroviral agents (NRTIs, NNRTIs, and PIs). HIV-1 integrase is essential for replication of the virus as a virally encoded enzyme that integrates the viral DNA into the genome of the host cell. Inhibition of HIV-1 integrase prevents the two-step process of endonucleolytic removal of the terminal dinucleotide from each 3′end of the viral DNA followed by the covalent integration of the viral DNA, at these modified 3′ends, into the host DNA, thereby representing a viable intervention in the viral life cycle. In vitro, raltegravir inhibited the strand transfer activity of HIV-1 integrase with an IC50 of 2–7nM with > 1,000-fold selectivity over other phosphoryltransferases. In addition, its in vitro IC95 for HIV-1 in 10% fetal bovine serum and 50% human serum was 19 and 33 nM, respectively. Raltegravir was well tolerated with no dose-related toxicities and a safety profile comparable to placebo. The most common clinical adverse events were diarrhea, nausea, vomiting, fatigue, headache, flushing, pruritus, and injection-site reactions.
Originator
Merck (US)
The Uses of Raltegravir
Raltegravir (MK-0518, Isentress) is a potent integrase (IN) inhibitor for WT and S217Q PFV IN with IC50 of 90 nM and 40 nM, respectively.
The Uses of Raltegravir
Raltegravir (MK-0518) is a potent integrase (IN) inhibitor for WT and S217Q PFV IN.
Background
Raltegravir is an antiretroviral drug produced by Merck & Co., used to treat HIV infection. It received approval by the U.S. Food and Drug Administration (FDA) on 12 October 2007, the first of a new class of HIV drugs, the integrase inhibitors, to receive such approval.
Indications
For the treatment of HIV-1 infection in conjunction with other antiretrovirals.
What are the applications of Application
Raltegravir is Raltegravir is a potent integrase strand transfer inhibitor for (PFV) IN
Definition
ChEBI: Raltegravir is a pyrimidone that is pyrimidin-4(3H)-one in which the hydrogens at positions 2, 3, 5 and 6 are replaced by 2-[(5-methyl-1,3,4-oxadiazole-2-carbonyl)amino]propan-2-yl, methyl, hydroxy, and N-[(4-fluorophenyl)methyl]aminoacyl groups, respectively. It is an antiretroviral drug used for treatment of HIV infection. It has a role as an antiviral drug and a HIV-1 integrase inhibitor. It is a 1,2,4-oxadiazole, a dicarboxylic acid amide, a member of monofluorobenzenes, a pyrimidone, a hydroxypyrimidine and a secondary carboxamide.
brand name
Isentress
Acquired resistance
Several characteristic mutations leading to typical amino acid exchanges have been characterized in cell culture studies and confirmed in clinical trial participants with virological failure while receiving raltegravir in combination with other antiretrovirals. Virological failure has generally been associated with mutations at one of three residues – Y143, Q148 or N155 – usually in combination with at least one other mutation.
Pharmaceutical Applications
Formulated as the potassium salt for oral administration.
Pharmacokinetics
Oral absorption: Not known/available
Cmax 400 mg twice daily: c. 2.17 mg/L
Plasma half-life: c. 9 h
Volume of distribution: Not known/available
Plasma protein binding: c. 83%
Absorption and distribution
It may be administered without regard to food. There are few data regarding its capacity to penetrate into genital secretions or breast milk. A study of 25 HIV-infected individuals receiving raltegravir as a component of combination antiretroviral therapy found that 24 had detectable levels and that 50% of these reached a level exceeding the 95% inhibitory concentration reported to inhibit HIV-1 strains fully susceptible to integrase inhibition.
Metabolism and excretion
It is not a substrate, and does not appear to inhibit or induce the cytochrome P450 enzyme complex. It is primarily metabolized through hepatic glucuronidation mediated by the UGT-1A1 enzyme. It is excreted in the feces (51%) and the urine (32%) as unaltered compound and its glucuronide. There are no recommended dose adjustments for weight, sex and race, or for hepatic or renal insufficiency. The pharmacokinetic handling in children has not been determined.
Clinical Use
Treatment of HIV infection (in combination with other antiretroviral drugs)
Side Effects
Its toxicity profile to date is remarkably benign. Clinical trial participants experienced similar types and frequencies of adverse events as those receiving placebo. The most frequently reported adverse events were nausea, diarrhea and headache and were mostly mild to moderate in intensity. Myopathy, rhabdomyolysis and elevations of creatinine phosphokinase have been noted in a few trial participants and it should be used cautiously in combination with drugs associated with muscle toxicity.
Synthesis
The synthesis of raltegravir begins with the treatment of acetone cyanohydrin with liquid ammonia in a pressure vessel. The resulting aminonitrile is protected as the benzyl carbamate before reaction of the nitrile moiety with hydroxylamine to afford the amidoxime. The pyrimidone ring is then constructed by condensation with dimethyl acetylenedicarboxylate and subsequent cyclization in hot xylene. Methylation of the pyrimidone is performed next with iodomethane and magnesium methoxide in dimethylsulfoxide followed by conversion of the methyl ester to an amide with 4-fluorobenzylamine. The amine, liberated from hydrogenolytic removal of the carbobenzyloxy-protecting group, is acylated with oxadiazolecarbonyl chloride, prepared in three steps from 5-methyltetrazole, to afford raltegravir.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin,
consider increasing raltegravir dose.
Antivirals: avoid with fosamprenavir.
Orlistat: absorption of raltegravir possibly reduced.
Ulcer-healing drugs: concentration increased by
omeprazole and famotidine.
Metabolism
Hepatic (UGT1A1)
Metabolism
Metabolised via glucuronidation, catalysed by the enzyme uridine diphosphate glucuronosyltransferase. Raltegravir is excreted in both urine and faeces as unchanged drug and metabolites.
Properties of Raltegravir
| Melting point: | 216 °C(Solv: isopropanol (67-63-0)) |
| Density | 1.46±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Very Slightly) |
| form | Solid |
| pka | 4.50±1.00(Predicted) |
| color | White to Light Beige |
| CAS DataBase Reference | 518048-05-0(CAS DataBase Reference) |
Safety information for Raltegravir
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Raltegravir
Raltegravir manufacturer
ShreeGen Pharma Ltd (part of Rumit Group of Companies)
Equilife Laboratories Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
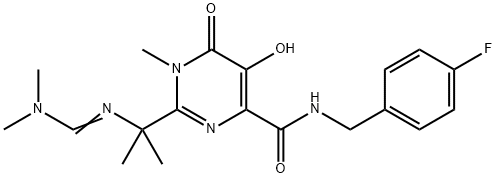
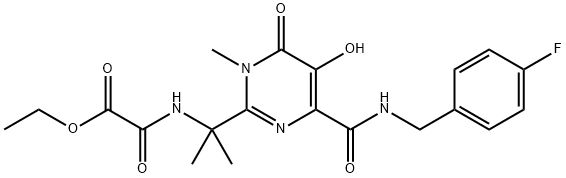
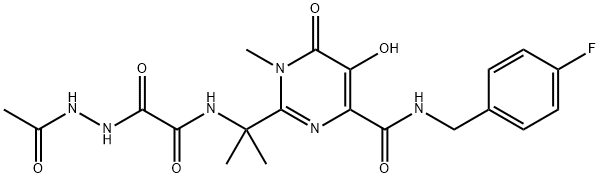
You may like
-
 518048-05-0 Raltegravir 98%View Details
518048-05-0 Raltegravir 98%View Details
518048-05-0 -
 518048-05-0 98%View Details
518048-05-0 98%View Details
518048-05-0 -
 Raltegravir 98%View Details
Raltegravir 98%View Details
518048-05-0 -
 Raltegravir 518048-05-0 98%View Details
Raltegravir 518048-05-0 98%View Details
518048-05-0 -
 Raltegravir 98% CAS 518048-05-0View Details
Raltegravir 98% CAS 518048-05-0View Details
518048-05-0 -
 Raltegravir CAS 518048-05-0View Details
Raltegravir CAS 518048-05-0View Details
518048-05-0 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1