Gold(III) chloride
Synonym(s):Trichlorogold
- CAS NO.:13453-07-1
- Empirical Formula: AuCl3
- Molecular Weight: 303.33
- MDL number: MFCD00011322
- EINECS: 236-623-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
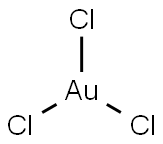
What is Gold(III) chloride?
Chemical properties
orange-red to dark red crystals
Physical properties
Red monoclinic crystals; deliquesces; density 4.7 g/cm3; sublimes at 180°C (760 torr); highly soluble in water; soluble in alcohol and ether; slightly soluble in liquid ammonia.
The Uses of Gold(III) chloride
Gold(III) chloride is used in colloidal gold solutions, in photography and as a print toning agent(gold toning), starting solution to form other gold compounds and a precursor for preparation of ultra pure gold metal. It is used in electroplating and electroless plating as an anode in an electric cell. Gold(III) chloride acts as a gold catalyst and cell body stains for bright field and dark field microscopy.
The Uses of Gold(III) chloride
Photography, gold plating, special inks, medicine, ceramics (enamels, gilding, and painting porcelain), glass (gilding, ruby glass), manufacture of finely divided gold and purple of Cassius.
The Uses of Gold(III) chloride
It is important to note that the Cl2 used here must be hot chlorine gas.
Preparation
Gold(III) chloride may be produced by the combination of metallic gold with chlorine gas at elevated temperatures:
2Au + 3Cl2 → 2AuCl3
It may be prepared in the laboratory by the reaction of iodine monochloride with metallic gold:
2Au + 6ICl → 2AuCl3 + 3I2
The compound should be stored tightly closed and protected from light.
Definition
A compound prepared by dissolving gold in aqua regia. The bright yellow crystals (chloroauric acid) produced on evaporation are heated to form dark red crystals of gold(III) chloride. The chloride decomposes easily (at 175°C) to give gold(I) chloride and chlorine; at higher temperatures it decomposes to give gold and chlorine. Gold(III) chloride is used in photography. It exists as a dimer, Au2Cl6.
Reactions
When heated at 254ºC, gold(III) chloride decomposes to gold(I) chloride and chlorine.
Passing hydrogen sulfide into an ether solution of the compound yields gold(III) sulfide, Au2S3.
A similar reaction occurs when alcoholic solutions of gold(III) chloride and hydrogen selenide are mixed, producing gold(III) selenide, Au2Se3, a black amorphous solid.
Gold(III) chloride may be reduced readily to metallic gold by common reducing agents. Thus, reduction with stannous chloride in dilute aqueous medium yields colloidal gold in which the atom carries a negative charge. “Cassius purple” is produced from the oxidation of tin to form H2Sn(OH)6, which protects colloidal gold from coagulation, imparting ruby red color to the solution.
Gold(III) chloride reacts with ammonia forming a gold(III)-nitrogen derivative, an explosive product, known as, “fulminate of gold”. Reaction with Grignard reagent, RMgX in ether yields dialkyl gold(III) chloride, R2AuCl3, which may be converted readily to other dialkyl gold(III) complexes by replacement of the chloride anion by a donor ligand.
General Description
The structure of gold chloride is monoclinic in nature. It sublimes at elevated temperatures.
Safety Profile
Experimental reproductive effects. Human mutation data reported. Reaction with ammonia or ammonium salts yields fulminating gold, a heat-, friction-, and impact-sensitive explosive similar to mercury and silver fulminates. See also GOLD COMPOUNDS and CHLORIDES. When heated to decomposition it emits toxic fumes of Cl-.
Properties of Gold(III) chloride
| Melting point: | 254°C |
| Boiling point: | 265 °C |
| Density | 3.9 g/mL at 25 °C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO (Sparingly), Methanol (Very Slightly) |
| form | Crystalline Powder, Crystals or Chunks |
| color | yellow |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,4521 |
| Solubility Product Constant (Ksp) | pKsp: 24.5 |
| CAS DataBase Reference | 13453-07-1(CAS DataBase Reference) |
| EPA Substance Registry System | Gold chloride (AuCl3) (13453-07-1) |
Safety information for Gold(III) chloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Gold(III) chloride
Gold(III) chloride manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)



You may like
-
 Auric chloride 99%View Details
Auric chloride 99%View Details -
 Tetrachloroauric acid 99%View Details
Tetrachloroauric acid 99%View Details -
 Gold(III) Chloride CAS 13453-07-1View Details
Gold(III) Chloride CAS 13453-07-1View Details
13453-07-1 -
 Gold(III) chloride CAS 13453-07-1View Details
Gold(III) chloride CAS 13453-07-1View Details
13453-07-1 -
 Gold(III) chloride CAS 13453-07-1View Details
Gold(III) chloride CAS 13453-07-1View Details
13453-07-1 -
 Gold(III) chloride CAS 13453-07-1View Details
Gold(III) chloride CAS 13453-07-1View Details
13453-07-1 -
 Gold(III) chloride CAS 13453-07-1View Details
Gold(III) chloride CAS 13453-07-1View Details
13453-07-1 -
 Gold(III) chloride, 99.99% (au 64.4% min) CAS 13453-07-1View Details
Gold(III) chloride, 99.99% (au 64.4% min) CAS 13453-07-1View Details
13453-07-1