Thionyl chloride
Synonym(s):Sulfurous acid dichloride, Sulfurous oxychloride;Thionyl chloride
- CAS NO.:7719-09-7
- Empirical Formula: Cl2OS
- Molecular Weight: 118.97
- MDL number: MFCD00011449
- EINECS: 231-748-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
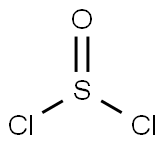
What is Thionyl chloride?
Chemical properties
Thionyl chloride is a pale yellow to reddish liquid. Suffocating odor like sulfur dioxide. Fumes form when exposed to moist air.
Description
Thionyl chloride can be used as a chlorinating agent, an esterifying agent or a dehydrating agent depending upon the reactants and the reactant conditions. As a chlorinating agent, Mobay thionyl chloride selectively replaces aliphatic hydroxyl groups without attacking double bonds or other functional groups.
Physical properties
Pale yellow to red fuming liquid; suffocating odor; refractive index 1.517 at 20°C; density 1.631 g/mL at 20°C; freezes at -101°C; boils at 75.6°C; decomposes at 140°C; decomposes in water; soluble in benzene, chloroform, and carbon tetrachloride.
The Uses of Thionyl chloride
Thionyl chloride (SOCl2) is used as a chlorinating agent in manufacturing organic compounds. It is also used as a solvent in high-energy lithium batteries. Thionyl chloride can replace OH or SH groups with chlorine atoms; reacts with Grignard reagents to form the corresponding sulfoxides, in the synthesis of herbicides, surfactants, drugs, vitamins, and dyestuffs.
The Uses of Thionyl chloride

A mixture of the SM (1.0 g, 6.3 mmol) in ACN (20 mL) was treated with SOCl2 (0.8 mL). The resulting mixture was refluxed for 2 h. The solvent was removed in vacuo to provide the crude product as an oil which was used in the next step without further purification.
Preparation
Sulfurous oxychloride can be prepared by oxidation of sulfur dichloride with sulfur trioxide: SCl2 + SO3 → SOCl2 + SO2
Also, the compound can be prepared by reacting sulfur dioxide with phosphorus pentachloride: SO2 + PCl5 → SOCl2 + POCl3.
Definition
ChEBI: Thionyl chloride is a sulfinyl halide in which both of the halide atoms are chorines. It is a sulfinyl halide and a chlorine molecular entity.
General Description
Thionyl chloride appears as a colorless to yellow fuming liquid with a suffocating pungent odor. Boiling point 79 °C. A lachrymator. Highly corrosive and toxic. Long-term inhalation of low concentrations or short-term inhalation of high concentrations has adverse health effects.
Reactivity Profile
Thionyl chloride reacts, potentially explosively, with dimethyl sulfoxide or dimethylformamide containing traces of iron or zinc [Spitulnik, M. J., Chem. Eng. News, 1977, 55(31), p. 31]. Undergoes violent reactions with bases (ammonia, sodium hydroxide, potassium hydroxide, amines), alkali metals (sodium, potassium), esters (ethyl acetate), toluene mixed with ethanol / water [Bretherick, 5th ed., 1995, p. 1325]. Has an expansion ratio from gas to liquid of nearly 1000:1. Hence may cause an explosion if heated while contained [MCA Case History No. 1808]. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Perchloric acid ignites on contact with sulfinyl chloride. (Bailar, 1973, Vol. 2, 1442). SOCl2 reacts with esters, such as ethyl acetate, forming toxic SO2 gas and water soluble/toxic acyl chlorides, catalyzed by Fe or Zn (Spagnuolo, C.J. et al. 1992. Chemical and Engineering News 70(22):2.).
Hazard
Strong irritant to skin, tissue, and upper respiratory tract.
Health Hazard
CORROSIVE and/or TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire will produce irritating, corrosive and/or toxic gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
EXCEPT FOR ACETIC ANHYDRIDE (UN1715), THAT IS FLAMMABLE, some of these materials may burn, but none ignite readily. May ignite combustibles (wood, paper, oil, clothing, etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Flammable/toxic gases may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. Substance may be transported in a molten form.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by inhalation. The material itself is more toxic than sulfur dioxide. Has a pungent odor similar to that of sulfur dioxide; it fumes upon exposure to air. Violent reaction with water releases hydrogen chloride and sulfur dioxide. Both these decomposition products constitute serious toxicity hazards. A corrosive irritant that causes burns to the skin and eyes. A powerful chlorinating agent. Potentially explosive reaction with ammonia, bis(dimethy1amino)sulfoxide - (above 80℃), chloryl perchlorate, 1,2,3- cyclohexanetrione trioxime + sulfur dioxide, dimethyl sulfoxide, hexafluoroisopropylideneaminolithium. Violent reaction or ignition with 2,4-hexadiyn-1-6-di01, onitrobenzoyl acetic acid, o-nitrophenylacetic acid, sodum (ignites at 300℃). Incompatible with ammonia, dimethyl formamide + trace iron or zinc, linseed oil + quinoline, toluene + ethanol + water. When heated to decomposition it emits toxic fumes of SOx and Cl-. See also HYDROGEN CHLORIDE and SULFUR DIOXIDE.
Potential Exposure
Thionyl chloride is used as specialty chlorinating agent, particularly in preparation of organic acid chlorides; in organic synthesis; as a catalyst.
Shipping
UN1836 Thionyl chloride, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Crude SOCl2 can be freed from sulfuryl chloride, sulfur monochloride and sulfur dichloride by refluxing it with sulfur and then fractionally distilling twice. [The SOCl2 is converted to SO2 and sulfur chlorides. The S2Cl2 (b 135.6o) is left in the residue, whereas SCl2 (b 59o) passes over in the forerun.] The usual purification is to distil it from quinoline (50g SOCl2 to 10g quinoline) to remove acid impurities, followed by distillation from boiled linseed oil (50g SOCl2 to 20g of oil). Precautions must be taken to exclude moisture. Thionyl chloride is used extensively in organic syntheses and can be prepared by distillation of technical SOCl2 in the presence of diterpene (12g/250mL SOCl2), and avoiding overheating. Further purification is achieved by redistillation from linseed oil (1-2%) [Rigby Chem Ind (London) 1508 1969]. Gas chromatographically pure material is obtained by distillation from 10% (w/w) triphenyl phosphite [Friedman & Wetter J Chem Soc (A) 36 1967, Larsen et al. J Am Chem Soc 108 6950 1986]. HARMFUL VAPOURS.
Incompatibilities
Reacts violently with water releasing sulfur dioxide and hydrogen chloride. Keep away from water, acids, alcohols, alkalis, ammonia, chloryl perchlorate.
Waste Disposal
Spray on a thick layer of a (1:1) mixture of dry soda ash and slaked lime behind a shield. After mixing, spray water from an atomizer with great precaution. Transfer slowly into a large amount of water. Neutralize and drain into the sewer with sufficient water.
Properties of Thionyl chloride
| Melting point: | -105 °C |
| Boiling point: | 79 °C(lit.) |
| Density | 1.64 g/mL at 20 °C |
| vapor density | 4.1 (vs air) |
| vapor pressure | 97 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 105°C |
| storage temp. | Store at RT. |
| solubility | Miscible with toluene, chloroform, benzene, carbon tetrachloride and diethyl ether. |
| form | Liquid |
| appearance | Clear liquid |
| color | ≤50(APHA) |
| Odor | Characteristic, pungent odor |
| Water Solubility | REACTS |
| Sensitive | Moisture Sensitive |
| Merck | 14,9348 |
| Exposure limits | ACGIH: TWA 50 ppm OSHA: TWA 25 ppm; STEL 125 ppm NIOSH: IDLH 2300 ppm |
| Dielectric constant | 9.1(22℃) |
| Stability: | Reacts violently with water. Incompatible with most common metals, strong reducing agents, strong bases, alcohols, amines. |
| CAS DataBase Reference | 7719-09-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Thionyl chloride(7719-09-7) |
| EPA Substance Registry System | Thionyl chloride (7719-09-7) |
Safety information for Thionyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H331:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Thionyl chloride
Thionyl chloride manufacturer
JSK Chemicals
Aashi Chem
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Thionyl chloride supplierView Details
Thionyl chloride supplierView Details
7719-09-7 -
 Thionyl Chloride 98%View Details
Thionyl Chloride 98%View Details -
 Thionyl Chloride 99%View Details
Thionyl Chloride 99%View Details -
 THIONYL CHLORIDE 2125597 99%View Details
THIONYL CHLORIDE 2125597 99%View Details
2125597 -
 Thionyl chloride CAS 7719-09-7View Details
Thionyl chloride CAS 7719-09-7View Details
7719-09-7 -
 Thionyl Chloride CASView Details
Thionyl Chloride CASView Details -
 THIONYL CHLORIDE For Synthesis CASView Details
THIONYL CHLORIDE For Synthesis CASView Details -
 Thionyl Chloride Liquid, 300KgView Details
Thionyl Chloride Liquid, 300KgView Details
7719-09-7
