Glycocholic acid
Synonym(s):3α,7α,12α-Trihydroxy-5β-cholan-24-oic acid N-(carboxymethyl)amide;Cholylglycine;N-(3α,7α,12α-Trihydroxy-24-oxocholan-24-yl)-glycine
- CAS NO.:475-31-0
- Empirical Formula: C26H43NO6
- Molecular Weight: 465.63
- MDL number: MFCD00065902
- EINECS: 207-494-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-26 17:46:08
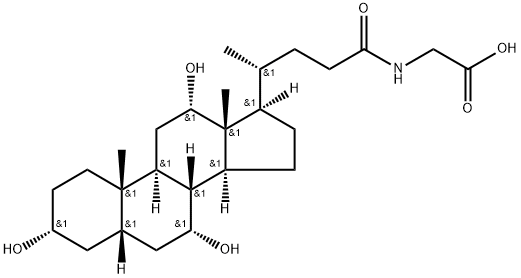
What is Glycocholic acid?
Description
Glycocholic acid is a glycine-conjugated form of the primary bile acid cholic acid and has roles in the emulsification of fats. It reduces expression of the gene encoding the farnesoid X receptor (FXR) and increases expression of the genes encoding the bile acid receptors TGR5 and S1PR2 in SNU-245 cells when used at a concentration of 1.6 μmol/ml. Glycocholic acid (250 μM) increases the intracellular accumulation and cytotoxicity of epirubicin in Caco-2 cells, as well as decreases expression of the genes encoding multidrug resistance protein 1 (MDR1), MDR-associated protein 1 (MRP1), and MRP2 when used alone or in combination with epirubicin. It increases absorption of epirubicin into everted sacs of rat ileum and jejunum when used at a concentration of 250 μM. The bile acid composition ratio of glycocholic acid is elevated in bile of patients with cholangiocarcinoma compared with patients with pancreatic cancer or benign biliary diseases. Serum levels of glycocholic acid are elevated in patients with hepatocellular carcinoma compared with healthy individuals.
Description
N-Cholylglycine. Bile salt, conjugate of cholate and glycine, usually as the sodium salt. It acts as a detergent to solubilize fats for absorption and is itself absorbed. It is used as a cholagogue and choleretic.
Chemical properties
Off-White Solid
The Uses of Glycocholic acid
Labelled Glycocholic Acid. The product of conjugation of cholic acid with glycine; chief ingredient of the bile of herbivorous animals. In the weakly alkaline bile fluid glycocholic acid exists as the sodium salt.
What are the applications of Application
Glycocholic acid is a conjugate of cholic acid and glycine
Definition
ChEBI: A bile acid glycine conjugate having cholic acid as the bile acid component.
Purification Methods
Glycocholic acid crystallises from hot water as the sesquihydrate. Dry it at 110o in vacuo. An analytical sample is prepared by suspending the acid (4g) in H2O (400mL) at ~20o, heating to boiling with slow stirring, filtering hot and allowing to cool to ~20o. The acid is filtered off, washed with H2O, dried in air, recrystallised from 5% aqueous EtOH, washed well and dried over P2O5 in a moderate vacuum to constant weight. Recrystallisation from EtOH/EtOAc, and drying, gave the anhydrous acid. [Cortese & Bauman J Am Chem Soc 57 1393 1935, Bergstrom & Norman Acta Chem Scand 7 1126 1953, Beilstein 10 IV 2077.]
Properties of Glycocholic acid
| Melting point: | 128°C |
| Boiling point: | 568.76°C (rough estimate) |
| Density | 1.1336 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Refrigerator |
| solubility | methanol: 0.1 g/mL, clear, colorless |
| form | Solid |
| pka | 4.4(at 25℃) |
| color | White to Off-White |
| Water Solubility | 329.9mg/L(20 ºC) |
| Merck | 13,4507 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 475-31-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Glycocholic acid(475-31-0) |
Safety information for Glycocholic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Glycocholic acid
| InChIKey | RFDAIACWWDREDC-NSPZZGDONA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Glycocholic acid 95% (HPLC) CAS 475-31-0View Details
Glycocholic acid 95% (HPLC) CAS 475-31-0View Details
475-31-0 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1