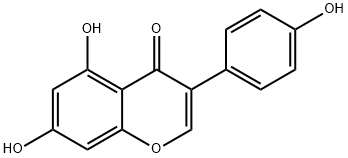
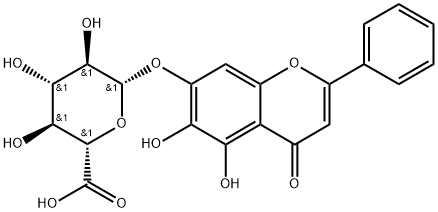
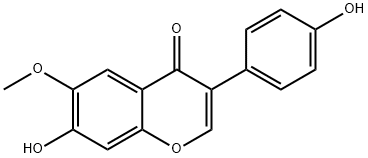
Genistin
Synonym(s):4′,5,7-Trihydroxyisoflavone 7-glucoside;Genistein 7-glucoside;Genistein, 7-O-β-D-Glucopyranoside;Genistein-7-O-β-D -glucopyranoside;Genistoside
- CAS NO.:529-59-9
- Empirical Formula: C21H20O10
- Molecular Weight: 432.38
- MDL number: MFCD00016883
- EINECS: 610-921-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 19:45:46
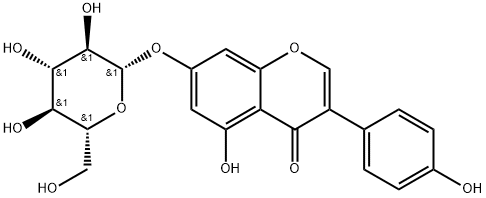
What is Genistin?
Description
Genistin is a natural isoflavone isolated from legumes, including soy and kudzu. It is a phytoestrogen, as it stimulates the growth of estrogen-
Chemical properties
White Powder
The Uses of Genistin
Genistin has been used for immune reactivity against influenza viruses in vitro and as an internal standard in isoflavones quantification.
The Uses of Genistin
A derivative of Genistein. Inhibitor
The Uses of Genistin
An isoflavone glycoside which is an inactive analog of the PTK inhibitor Genistein.
The Uses of Genistin
Glucoside of genistein that inhibits protein tyrosine kinase
What are the applications of Application
Genistin is an isoflavone glycoside which is an inactive analog of the PTK inhibitor Genistein
Definition
ChEBI: Genistein 7-O-beta-D-glucoside is a 7-hydroxyisoflavones 7-O-beta-D-glucoside. It is functionally related to a genistein. It is a conjugate acid of a genistein 7-O-beta-D-glucoside(1-).
General Description
Genistin belongs to the class of isoflavone glycosides generally extracted from soybeans.
Biochem/physiol Actions
Selective inhibitor of mammalian terminal deoxynucleotidyl transferase (TdT), with no measurable effect on mammalian or microbial DNA polymerases.
Purification Methods
Genistin is repeatedly crystallised from hot 80% EtOH/water and treated with charcoal (Nuchar) until free from saponin. The presence of saponin is detected by adding crystals to conc H2SO4 when the citron yellow colour changes to red, then purple. Pure genistin does not change colour. UV in 85% EtOH has max at 262.5nm. [Walter J Am Chem Soc 63 3273 1941, Beilstein 18 III/IV 2732.]
References
1) Uchiyama et al. (2005), Selective inhibitors of terminal deoxyribonucleotidyltransferase (TdT): baicalin and genistin; Biochim. Biophys. Acta, 1725 298 2) Choi et al. (2007), Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells; Life Sci, 80 1403 3) Russo et al. (2006), Genistin inhibits UV light-induced plasmid DNA damage and cell growth in human melanoma cells; J. Nutr. Biochem., 17 103
Properties of Genistin
| Melting point: | 254°C |
| Boiling point: | 256 °C |
| alpha | D21 -28° (c = 0.6 in 0.02N NaOH); D26 -21.4° (pyridine) |
| Density | 1.642±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: 10 mg/mL |
| form | neat |
| pka | 6.12±0.20(Predicted) |
| form | Solid |
| color | Off-white |
| Merck | 13,4402 |
| BRN | 64479 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 week. |
Safety information for Genistin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Genistin
| InChIKey | ZCOLJUOHXJRHDI-CMWLGVBASA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Genistin CAS 529-59-9View Details
Genistin CAS 529-59-9View Details
529-59-9 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8