Bengenin
Synonym(s):Ardisic acid B;Cuscutin;Vakerin
- CAS NO.:477-90-7
- Empirical Formula: C14H16O9
- Molecular Weight: 328.27
- MDL number: MFCD00133120
- EINECS: 803-760-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
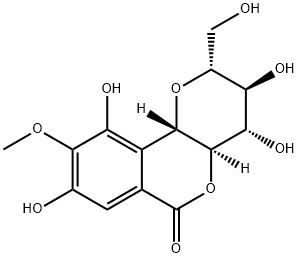
What is Bengenin?
Description
The plant resources of bergenin are rich, mainly from Rock cabbage (Bergenia purpurascens) and thick leafy rock cabbage (Astilbe macroflora) from Saxifragaceae
and the roots, stems, and leaves of bai liang jin (Ardisia crispa) from Ardisia japonica (Thunb.) Blume. In Chinese medicine Rock cabbage (Bergenia purpurascens) is
the common folk medicine, with nourishing, stanching bleeding, relieving a cough,
and other effects. Its earliest record is contained in the classified herbal in Qing
Dynasty .
There are ten kinds of Rock cabbage (Bergenia purpurascens) in Asia, mainly
growing in East Asia, north of South Asia, and southeastern Central Asia. There are
seven species (including three endemic species) in China, mainly distributed in
Shaanxi (Qinling), Xinjiang, Sichuan, Yunnan, and Tibet .
Chemical properties
White powder
Physical properties
Appearance: white, loose needle-like crystal or crystalline powder with light odor and bitter taste and discolored when encountered with light or heat. Solubility: dissolved in methanol and slightly soluble in water or ethanol. Melting point: 232– 240?°C. Specific optical rotation: ?38° to ?45°.
History
In 1958, Hay and Haynes first reported the complete synthesis of bergenin, using
4-methoxy gallate and A-D-bromo-2, 3, 4, 6-tetraacetylglucose as raw materials to
synthesize bergenin successfully .
In 1987, Chen Wendou determined the content of bergenin as 5.8% in the alcohol
extract of Astilbe chinensis (Maxim.), Franch. et Savat.(Luoxinfu), and Rock cabbage (Bergenia purpurascens) by using high-performance liquid chromatography.
In 1991, Wang Junping determined the content of bergenin in the alcohol extract of
Rodgersia podophylla as 19.17% with spectrophotometry.
By doing thin layer qualitative analysis, Liu Nunian discovered that bergenin
could be found in seven kinds of plants including sour moss (Ardisia solanacea),
Luosan tree (Ardisia quinquegona Bl.), Cinnabar root (Ardisia crenata Sims),
Zijinniu (Ardisia japonica), Lianzuozijinniu (Ardisia primulaefolia), Jiujielong
(Ardisia pusilla), and Xinyezijinniu (Ardisia maclurei) from Ardisia. In addition,
Zhang Yi determined the content of bergenin in the medicinal plants from Ardisiawith high-performance liquid chromatography, and results showed that there
were four species of plants with the content of bergenin of more than 1%, having
great resource utilization value. There are 11 species of plants with the content of
bergenin less than 1%, such as Xiaoqiaomuzijinniu (Ardisia arborescens), twist
fruit, fine umbrella (Ardisia affinis Hemsl.), and so on. They had some useful
value as well .
In recent years, it has been found that both 8, 10-dimethylca bergenin and
11-0-gallate betaine had weak inhibitory effects on the tumor in mice, and their
effects were stronger than that of bergenin. The effect of acetylated bergenin was
stronger than that of bergenin on liver injury induced by chloroform in mice. The
new bergenin derivatives from the Ardisia gigantifolia stapf had a certain degree of
free radical-scavenging effect and anti-HIV viral activity .
The Uses of Bengenin
A herbal remedy with antioxidant, antimicrobial and antiinflammatory properties.
What are the applications of Application
Bergenin monohydrate is an isocoumarin
Definition
ChEBI: A natural product found in Cenostigma gardnerianum.
General Description
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG
Biochem/physiol Actions
Bergenin displays antinociceptive and anti-inflammatory properties, in addition to its use in folk medicines as a potent hepatoprotective agent. Bergenin acts by inhibiting the formation of interleukin 1beta and TNF-α, which suggests its potential use for controling inflammatory pain.
Pharmacology
1. Cough-relieving effect
Bergenin had a significant relieving effect on coughs caused by electrical stimulation of the laryngeal nerve and ammonia spray. The dose of its cough-relieving
effect was equivalent to 1/7–1/4 dose of codeine. No tolerance was found after a
23-day consecutive administration of bergenin due to its selective inhibition on
cough center .
2. Expectorant effect
Dwarf tea (Japanese Ardisia Herb) decoction was found to have a clear expectorant effect after intragastric administration to mice at the dose of 25?g/kg, and its
effect strength is equivalent to that of Platycodon grandiflorum at the same dose.
3. Anti-inflammatory and anti-ulcer effect
In the model of chronic bronchitis caused by sulfur dioxide fumigation in rats,
80 mg/kg/day of bergenin was given once a day for 10?days. The results showed that
the tracheal goblet cell number from the rats in the treatment group decreased, suggesting that the amount of sputum reduced. Inflammatory cell infiltration was found
to alleviate, and the degree of emphysema and lung collapse were also reduced.
Okada found that bergenin had a therapeutic effect on experimental gastric ulcer
rats, consistent with the clinical application of bergenin in the treatment of gastric
ulcer, duodenal ulcer, and chronic gastritis .
Recent studies have shown that bergenin had certain analgesic and antiinflammatory properties and had the potential to control inflammatory pain through
inhibiting the production of IL-β and TNF-α . Bergenin preparations have been
widely used clinically for the treatment of chronic bronchitis, gastritis, and gastric
and duodenal ulcers.
4. Antipyretic effect
The alcohol extracts of Ardisia crispa had a strong antipyretic effect on the fever
caused by the injection of cholera typhoid, paratyphoid, TAB vaccine, and tetanus
toxin in the ear of rabbit.
5. Antiviral effect
Rodgersia podophylla alcohol extract at the dose of 0.017–0.034?mg/ml cannot
only inhibit the replication of DNA virus but also the replication of RNA virus. The
cells with inactivated virus can continue to split and proliferate, indicating that the
treatment is effective and with no obvious cell toxicity .
6. Liver protection
Bergenin was found to improve the liver injury induced by carbon tetrachloride
in mice. It cannot only reduce the release of glutamate acetyltransferase and sorbitol
dehydrogenase from mice liver but also reduce the glutathione reductase and
increase the glutathione content, indicating that bergenin protected the liver through
the regulation of glutathione and inhibiting the release of free radicals .
7. Cardiovascular system effects
Bergenin had some therapeutic effects on the arrhythmia in mice.
8. Toxicity test
Mice were intraperitoneally injected with bergenin; the minimum lethal dose is
10?g/kg; no toxic reaction was found after intragastric administration of bergenin at
the dose of 12?g/kg. No effects were found in the growth and development, liver
function, and ECG of young rats after the intragastric administration with bergenin
at the dose of 2.5?g/kg for consecutive 60?days, and no toxic performance was found
in the heart, liver, kidney, lung, spleen, stomach, intestine, brain, and other organs
by pathological biopsy
Clinical Use
Bergenin was recorded in People’s Republic of China Pharmacopoeia as an antitussive expectorant, used for the treatment of chronic bronchitis, emphysema, bronchial asthma, and other respiratory diseases. Used alone, or in compound preparation, such as compound bergenin tablets, Qingjin syrup, Compound Hu ear tablets, Qingfei antitussive syrup, liver poison net particles, Kechuanping oral solution, and silicone lung oral solution, it is mainly used for the treatment of chronic bronchitis. Its efficacy was proven to be relatively stable and improved in the compound preparations after a wide range of clinical validation.
Properties of Bengenin
| Melting point: | 237-240 °C(lit.) |
| Boiling point: | 386.03°C (rough estimate) |
| alpha | D18 -37.7° (c = 1.96 in ethanol); D24 -45.3° (c = 0.51 for anhydr in water) |
| Density | 1.3901 (rough estimate) |
| refractive index | 1.6550 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 8.26±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| λmax | 277nm(EtOH)(lit.) |
| CAS DataBase Reference | 477-90-7(CAS DataBase Reference) |
Safety information for Bengenin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bengenin
New Products
Tetrabutylammonium iodide (3,3-DIFLUOROCYCLOBUTYL)METHANOL 4,4-DIFLUOROCYCLOHEXANAMINE Cyclobutylamine (S)-3-Fluoro-pyrrolidine hydrochloride 3-Oxocyclobutanecarboxylic acid N-Hydroxy-2-methylpropanimidamide L-tert-Leucine,97% 2-Bromophenylacetonitrile, 97% Aluminum oxide, basic Calcium hydroxide, 95% Diallylamine, 99% 2-Iodobenzoic Acid 3-Methoxybenzonitrile Pentachlorobenzonitrile Chloral Dibenzoyl Peroxide Titanium Dioxide O-Benzylhydroxylamine Hydrochloride 2-Nitrobenzaldehyde 2-Picolylamine (2-Aminomethylpyridine) 2-Venylpyridine Ethyl-2-Chloroacetoacetate 4-Dimethylamine PyridineRelated products of tetrahydrofuran


You may like
-
 Bergenin CAS 477-90-7View Details
Bergenin CAS 477-90-7View Details
477-90-7 -
 Bergenin 95% CAS 477-90-7View Details
Bergenin 95% CAS 477-90-7View Details
477-90-7 -
 Bergenin 98% (HPLC) CAS 477-90-7View Details
Bergenin 98% (HPLC) CAS 477-90-7View Details
477-90-7 -
 Bergenin CAS 477-90-7View Details
Bergenin CAS 477-90-7View Details
477-90-7 -
 1126-74-5 3-Pyridineacrylic acid 98+View Details
1126-74-5 3-Pyridineacrylic acid 98+View Details
1126-74-5 -
 tert-Butyl carbazate 98+View Details
tert-Butyl carbazate 98+View Details
870-46-2 -
 TETRABUTYLAMMONIUM CYANIDE 10442-39-4 98+View Details
TETRABUTYLAMMONIUM CYANIDE 10442-39-4 98+View Details
10442-39-4 -
 19752-84-2 TETRAHYDRO-2H-PYRAN-3-OL 98+View Details
19752-84-2 TETRAHYDRO-2H-PYRAN-3-OL 98+View Details
19752-84-2
